
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
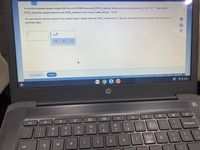
Transcribed Image Text:A chemistry graduate student Is given 300. mL of a 0.10M nitrous acid (HNO,) solution. Nitrous acid Is a weak acld with K,=4.5× 10 ". What mass of
-4
D.
KNO,
should the student dissolve In the HNO, solution to turn it Into a buffer with pH =3.15?
You may assume that the volume of the solution doesn't change when the KNO, is dissolved in it. Be sure your answer has a unít symbol, and round it to 2
significant diglits.
do
Ar
x10
Explanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Accessibility
O 10:47
hp
esc
backspace
%
!
#3
7
8
9.
6.
1
{
y
W
e
r
tab
%3D
%24
4.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemistry graduate student is given 450. mL of a 1.80M chlorous acid (HCIO,) solution. Chlorous acid is a weak acid with K= 1.1 × 10 . What mass of KC1O, should the student dissolve in the HC1O, solution to turn it into a buffer with pH = 1.92? You may assume that the volume of the solution doesn't change when the KCIO, is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits.arrow_forwardA chemistry graduate student is given 125. mL of a 1.30M dimethylamine ((CH,) NH solution. Dimethylamine is a weak base with K,=5.4 × 10 . What mass of (CH,), NH, Cl should the student dissolve in the (CH,) NH solution to turn it into a buffer with pH =10.80? You may assume that the volume of the solution doesn't change when the (CH, NH, Cl is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits. 10arrow_forwardAMoving to åñðt Question 15 The molar solubility of Pbl2, determined experimentally, is 3.6 x 10³ mol/L. This means that in 1.0 L of a saturated Pbl2 solution, only 3.6 x 10³ mol of Pbl2 dissolves, forming. mol of Pb2* lon and mol of l' ion. O 3.6 x 103, 7.2 x 103 O 3.6 x 103, 3.6 x 103 O 3.6; 3.6 O 1.29 x 10-3, 1.29 x 103 Question 15 of 22 > A Moving to another question will save this response. MacBook Pro F10 F7 esc F3 #3 $4 3 4 6 7 9 1 Q W E T Y F G K C V B commandarrow_forward
- A chemistry graduate student is given 450. mL of a 1.10M dimethylamine (CH.) NH) solution. Dimethylamine is a weak base with K,=5.4 × 10 4. What mass of (CH,),NH,CI should the student dissolve in the (CH,), NH solution to turn it into a buffer with pH = 10.89? You may assume that the volume of the solution doesn't change when the (CH,),NH,CI is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits. ?arrow_forwardIf 100.0 g calcium phosphate (Ksp= 1.3 x 10^-32) are placed in enough water to generate 750.0 mL of solution, calculate the molar solubility of the calcium phosphate solution and the molar solubility of the calcium ion if 30.0 mL of 0.400 M calcium nitrate solution is added to the original solution.arrow_forwardA chemistry graduate student is given 300. mL of a 1.30M hydrocyanic acid (HCN) solution. Hydrocyanic acid is a weak acid with K = 4.9 × 10 mass of KCN should the student dissolve in the HCN solution to turn it into a buffer with pH=9.51? -10 . What You may assume that the volume of the solution doesn't change when the KCN is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits. Explanation 81°F Mostly sunny Check $ 0.0 X с с % S F6 Q Search F7 FB F9 F10 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | A e c (4) D F11 *+ D F12 PrtSc Insert Deletearrow_forward
- A chemistry graduate student is given 450. mL of a 0.60M propanoic acid (HC,H,CO,) solution. Propanoic acid is a weak acid with K,=1.3 × 10 °. What mass of KC, H,CO, should the student dissolve in the HC,H,CO, solution to turn it into a buffer with pH =4.80? You may assume that the volume of the solution doesn't change when the KC,H,CO, is dissolved in it. Be sure your answer has a unit symbol, and round it to 2. 2 significant digits. alo х10 Ar Explanation Check © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use| Privacy Center | Accessibility ................ . ..................... .....arrow_forwardA chemistry graduate student is given 250. mL of a 1.80 M dimethylamine ((CH3), NH) solution. Dimethylamine is a 2 -4 weak base with K = 5.4 × 10 What mass of (CH, NH,Br should the student dissolve in the (CH,) NH solution to 2 turn it into a buffer with pH = 11.26? You may assume that the volume of the solution doesn't change when the (CH,) NH,Br is dissolved in it. Be sure your 2 answer has a unit symbol, and round it to 2 significant digits.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY