
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
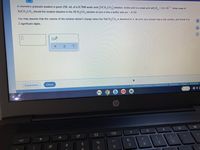
Transcribed Image Text:A chemistry graduate student Is glven 250. mL of a 0.70M acetic acld (HCH,CO,) solution. Acetic acld Is a weak acıd with K=1.8 × 10. What mass of
-5
NaCH, CO, should the student dissolve In the HCH,CO, solution to turn
D.
2.
It Into a buffer with pH =4.74?
You may assume that the volume of the solution doesn't change when the NaCH,CO, Is dissolved in it. Be sure your answer has a unit symbol, and round it to
2 significant digits.
dla
Ar
Explanation
Check
2021 McGraw-Hill Education. All Rights Reserved. Tems of Use Privacy Accessibility
hp
火
->
esc
&
#
9.
C@
4
00
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Marble is almost pure CaCO3. Acid rain has a devastating effect on marble statuary left outdoors. Assume that the reaction which occurs is CoCO3(s)+ H+(aq)Ca2+(aq)+HCO3(aq) Neglecting all other competing equilibria and using Tables 15.1 and 13.2, calculate (a) K for the reaction. (b) the molar solubility of CaCO3 in pure water. (c) the molar solubility of CaCO3 in acid rainwater with a pH of 4.00.arrow_forwardYou want to make a buffer with a pH of 10.00 from NH4+/NH3. (a) What must the [ NH4+ ]/[ NH3 ]ratio be? (b) How many moles of NH4Cl must be added to 465 mL of an aqueous solution of 1.24 M NH3 to give this pH? (c) How many milliliters of 0.236 M NH3 must be added to 2.08 g of NH4Cl to give this pH? (d) What volume of 0.499 M NH3 must be added to 395 mL, of 0.109 M NH4Cl to give this pH?arrow_forwardA buffer solution with it pH of 12.00 consists of Na3PO4 and Na2HPO4. The volume of solution is 200.0 mL. (a) Which component of the buffer is present in a larger amount? (b) If the concentration of Na3PO4 is 0.400 M, what mass of Na2HPO4 is present? (c) Which component of the buffer must be added to change the pH to 12.25? What mass of that component is required?arrow_forward
- A saturated solution of copper(II) iodate in pure water has a copper-ion concentration of 2.7 103 M. a What is the molar solubility of copper iodate in a 0.35 M potassium iodate solution? b What is the molar solubility of copper iodate in a 0.35 M copper nitrate solution? c Should there be a difference in the answers to parts a and b? Why?arrow_forwardAn analytical chemist has a solution containing chloride ion, Cl. She decides to determine the amount of chloride ion in the solution by titrating 50.0 mL of this solution by 0.100 M AgNO3. As a way to indicate the endpoint of the titration, she added 1.00 g of potassium chromate, K2CrO4 (see Figure 17.5). As she slowly added the silver nitrate to the solution, a white precipitate formed. She continued the titration, with more white precipitate forming. Finally, the solution turned red, from another precipitate. The volume of the solution at this point was 60.3 mL. How many moles of chloride ion were there in the original solution? How many moles of chloride ion were there in the final solution? You may make any reasonable approximations.arrow_forwardConsider a 2.0-L aqueous solution of 4.17 M NH3, where 21.0 g of NH4Cl are dissolved. To this solution, 4.8 g of CaCl2 are added. (a) What is [OH-] before CaCl2 is added? (b) Will a precipitate form? (c) What is [Ca2+] after equilibrium is established?arrow_forward
- How would the solubility of calcium fluoride be affected by the presence of fluoride ion from another source? What is the solubility of calcium fluoride in a saturated solution of barium fluoride? How does this compare with the value of the solubility of calcium fluoride found in Example 17.4? Is this what you expect?arrow_forwardA saturated solution of lead iodate in pure water has an iodate-ion concentration of 8.0 105 M. a What is the molar solubility of lead iodate in a 0.15 M lead nitrate solution at the same temperature? b Should the molar solubility of lead iodate in part a be the same as, greater than, or less than that of lead iodate in pure water? Why?arrow_forwardLead(II) chromate, PbCrO4, was used as a yellow paint pigment (chrome yellow). When a solution is prepared that is 5.0 104 M in lead ion, Pb2, and 5.0 105 M in chromate ion, CrO42, would you expect some of the lead(II) chromate to precipitate?arrow_forward
- A buffer solution is prepared by dissolving 1.50 g each of benzoic acid, C6H5CO2H, and sodium benzoate, NaC6H5CO2, in 150.0 mL of solution. (a) What is the pH of this buffer solution? (b) Which buffer component must be added, and in what quantity, to change the pH to 4.00? (c) What quantity of 2.0 M NaOH or 2.0 M HCl must be added to the buffer to change the pH to 4.00?arrow_forwardCalculate the solubility (in grams per liter) of magnesium hydroxide in the following. (a) pure water (b) 0.041 M Ba(OH)2 (c) 0.0050 M MgCl2arrow_forwardCalculate the solubility of barium sulfate (Ksp = 1.1 1010) in (a) water. (b) a 0.10 M barium chloride solution.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning