
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
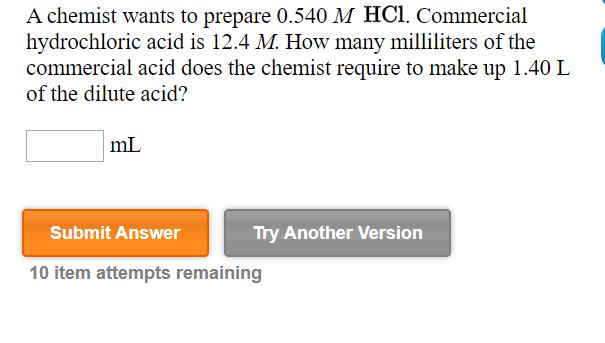
Transcribed Image Text:A chemist wants to prepare 0.540 M HC1. Commercial
hydrochloric acid is 12.4 M. How many milliliters of the
commercial acid does the chemist require to make up 1.40 L
of the dilute acid?
Submit Answer
Try Another Version
10 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- Q2.1 You know that Ka=1:8 x 103, what is the dissociation of 0.010 M acetic acid? a) 1.3 percent c) 4.2 percent b) 3.3 percent d) 206 percent. percentarrow_forwardQuestion 24 A solution is made by combining 4.00 g of sugar and 100 mL of water (density of water = 1.00 g/mL). What is the concentration in % w/w? (Hint: you can use density to convert from volume to mass) O26.0 4.00 0.0400 O 3.85 Question 25 MacBookarrow_forwardQuestion 1Carrow_forward
- [References] A person drinks 1.64x10 g of water per day. How many moles is this? n(H,O) = mol %3D Submit Answer Try Another Version 3 item attempts remainingarrow_forwardTest this weekend, these are some questions I'm struggling with. Checking back in my lessons now! 1. What is the empirical formula of an oxide containing 49.5% manganese? (Hint: Oxide = compound of Mn with O). Mn O Mass of element (in g) (assuming 100 g of compound) 1._49.5_ 2._50.5_ Moles of element 3.______ 4.______ Moles of element/Smallest moles 5.______ 6.______ Multiplier 7.______ 7.______ Empirical formula Mn2O7 2. What is the empirical formula of strontium chloride in problem 5? Sr Cl Mass of element (in g) 1. 2.21 2. 1.79 Moles of element 3.______ 4.______ Moles of element/Smallest moles 5.______ 6.______ Multiplier 7.______ 7.______ Empirical formula SrCl2 3. What is the empirical formula of an compound containing 0.830 % H; 45.8% Mn; 53.4% O? H Mn O Mass of element (in g) (assuming 100 g of compound) 1._0.830_ 2._45.8__ 3._53.4_ Moles of element 4.______ 5.______ 6.______ Moles of element/Smallest moles 7.______…arrow_forwardO CHEMICAL REACTIONS Finding chemical formulae from a mole ratio An unknown compound has the following chemical formula: PbOX where x stands for a whole number. Measurements also show that a certain sample of the unknown compound contains 7.70 mol of oxygen and 3.8 mol of lead. Write the complete chemical formula for the unknown compound. 0 Explanation Check 00 X S zz McGraw Hill LLC. All Rights Reserved. Termsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY