
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
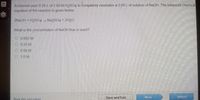
Transcribed Image Text:A chemist uses 0.25 Lof 2.00MH2SO4 to eompletely neutralize a 2.00 Lof solution of NaCH The balanced chemical
equation of the reaction is gven below.
2NAOH+H2S0O4 Naps04 2H00
What is the concentration of NaOH that is used?
O 0063 M
O 0 25 M
O 0 50 M
O 1.0M
Save and Exit
Next
Mark this and return
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 25.00 ml solution of acetic acid of unknown concentration is titrated with 0.1000 M KOH. The end point of the titration is found to equal 29.50 ml of KOH. What is the molarity of the unknown? O 0.08475 M O 0.05412 M O 0.4500 M O 0.1180 Marrow_forwardHow many ml of a 0.100M NaOH solution are required to fully react with 12.0 mL of a 0.150M H2SO4 solution?arrow_forwardHow can we complete the table from hypochlorous acid to sodium cynanide?arrow_forward
- You have a solution of NaOH of unknown concentration. A 15.98 mL sample of the NaOH solution was titrated with 0.377 M HNO3. It took 25.28 mL of HNO3 to reach the equivalence point. What was the concentration of the original NaOH solution?arrow_forwardIf 76.4 mL of 0.25 M lithium hydroxide are needed to completely neutralize 250.0 mL of a solution of sulfuric acid, what is the concentration of the sulfuric acid?arrow_forwardHow can we fill in the table ?arrow_forward
- Which of the following is considered both a double-replacement and neutralization reaction? KCIO3 KCIO + O2 C3H8 + 502 → 3CO2 + 4H2O 3Ca(NO3)2 + 2Na3PO4 – Ca3(PO4)2 + 6NANO3 2HCN + Sr(OH)2 → Sr(CN)2 + 2H2Oarrow_forwardA 0.261 g sample of NaHC2O4 (one acidic proton) required 17.5 mL of sodium hydroxide solution for complete reaction. Determine the molar concentration of the sodium hydroxide solution.arrow_forwardWhat is the molarity of an HCl solution if 42.8 mL of it is titrated to the equivalence point with 20.1 mL of a 1.07 M NaOH solution?arrow_forward
- You set up a titration in the lab to determine the concentration of an HCl solution. What is the molarity of an HCl solution if 0.0100 L of 0.245 M NaOH soln’ are required to neutralize 0.0150 L HCl? 2.25 M 0.163 M 1.34 M 0.245 Marrow_forwardA 50.0 mL sample of H2SO4 is neutralized with a KOH solution having a concentration of 0.400 M. If 22.6 mL of KOH are required to complete the neutralization, what is the concentration of the sulfuric acid solution?arrow_forwardthe ph of a solution is 5. what is the concentration of hydronium ion in the solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY