
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
A chemist measures the enthalpy change ΔH during the following reaction:
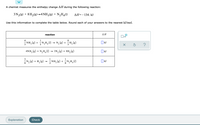
Transcribed Image Text:A chemist measures the enthalpy change AH during the following reaction:
3 N2(g) + 8 H2(g)→4 NH3(g) + N2H4(1)
ДН--134. kJ
Use this information to complete the table below. Round each of your answers to the nearest kJ/mol.
reaction
ΔΗ
x10
NH, 4) + N,H,() - N,6) + H, «)
8.
N,H, (1)
N, g) + -
3 H, (g)
O kJ
4NH, (g) + N,H, (1) → 3N, (g) + 8H, (g)
O kJ
3
1
1
,(8) + H, (g)
2 NH, (g) + -N.
8 N,H, (1)
8
kJ
Explanation
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Measurements show that the energy of a mixture of gaseous reactants increases by 301. kJ during a certain chemical reaction, which is carried out at a constant pressure. Furthermore, by carefully monitoring the volume change it is determined that – 104. kJ of work is done on the mixture during the reaction. Calculate the change in enthalpy of the gas mixture during the reaction. Be sure your answer has the correct number of significant digits. O exothermic ? Is the reaction exothermic or endothermic? O endothermicarrow_forwardGiven the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. AGran =? kJ 4A (g) + 2B (3) → 1C (g)+ 7D (g) AGi in kJ/mol Substance A (g) B (g) C(g) D (g) 76.34 + 5.24 - 55.86 - 14.55 O - 452.59 kJ/mol O + 452.59 kJ/mol O + 137.17 kJ/mol O - 141.51 kJ/mol O - 137.17 kJ/molarrow_forwardOne day while camping, a student used a propane stove to boil some water. The student heated 5.00 L of water from 15.6°C to 98.5°C. The molar enthalpy of combustion of propane is -2043.9 kJ/mol and heating the water required 1.38 mol of propane. Determine the efficiency of the propane camp stove if the heat released from the stove was used to heat the water. 62.4 % 38.4% D162 % 61.6%arrow_forward
- A chemist measures the enthalpy change AH during the following reaction: 2 H,O(1) + O2(9)→2 H,O2(1) ДН-196. kJ Use this information to complete the table below. Round each of your answers to the nearest kJ/mol. reaction ΔΗ x10 4H, 0, (1) → 4H,0(1) + 20, (g) kJ 2H,0, (1) → 2H,0(1) + 0, (g) I kJ 1 H,0(1) + 0,(2) - H,0,) H,0, (1) O kJarrow_forwardWhat mass (in g) of carbon monoxide must be burned to produce 170.6 kJ of heat under standard state conditions?arrow_forward(b) Calculate the enthalpy change for the reaction C(graphite) + 2 H2(g) → CH4(g) with the help of the enthalpy changes of the following reactions: C(graphite) + O2(g) → CO2(g) ∆H= -393.5 kJmol−1 H2(g) +1/2O2(g) → H2O(l) ∆H = -285.8 kJmol−1 CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(l) ∆H = -890.4 kJmol−1 The temperature is 25 ◦C in all instances.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY