
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Hh.190.
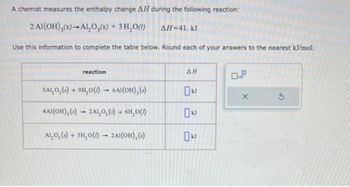
Transcribed Image Text:A chemist measures the enthalpy change AH during the following reaction:
2 Al(OH)3(s)-Al₂O3(s) + 3 H₂O(1) ΔΗ=41. kJ
Use this information to complete the table below. Round each of your answers to the nearest kJ/mol.
reaction
3ALO₂ (s) + 9H₂O()→ 6Al(OH), (s)
4AI(OH), (s)- 2AL₂O₂ (s) + 6H₂O(1)
ALO, (s) + 3H₂O() → 2A1(OH), (s)
1
AH
☐kJ
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (Q33) What is the root mean square speed (URMS) of fluorine gas at -36.7 °C?arrow_forwardIn the van der Waals equation there is a large difference in the "a" values for HF and Ne despite them having almost the same Molar Mass and similar size. Why is the "a" value for these so compounds so different? FULLY EXPLAIN esc ck Edit Format Table 12pt Paragraph Р 1 A N C B I U @ 2 W S #3 X E H D $ 4 R C 8 % 5 F ¡ T V MacBook Pro I ➡ A 6 G Y & 7. B H f U 0 words *00 8 J N ⠀⠀ ( 1 9 K M MOSISarrow_forwardHi! I am unsure of how to solve this problem. Thank you!arrow_forward
- Chemistry helpppp.arrow_forwardModule 7B- Stol G molar mass of ca V stemble Learning why can X U Clear Oval Hou 228/assignments/389/tasks/101 4/18 tasks attempted Based on the balanced reaction below, determine the number of grams of oxygen required to completely react with 3.40 g of propane, C3Hs C3HS (g) + 5 O2 (g) 3 CO2 (g) + 4 H20 (1) Mass of Oxygen Gas SAVE RESPONSE 7 18 1.116.0 PRIVACY POLICY TERMS OF USE CONTACT INFORMATION MacBook Pro G Search or type URL %23arrow_forwardAfter the following reaction is balanced: C₆H₁₂O₆ + O₂ →CO₂ + H₂O. What are the stoichiometric coefficients of C₆H₁₂O₆, O₂, CO₂ and H₂O, respectively.arrow_forward
- Suppose 125 g of NO3- flows into a swamp each day. What volume of N2 would be produced each day at 17.0°C and 1.00 atm if the denitrification process were complete?arrow_forwardb My Quest x Co. How to Ca x N NSU Logir x PeriodicTa x M Action Re x 0 mySig Tau x G4.25 ml to X NBA Final: x G scientific X NBA Final X c x + now.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=Dassignment-take [References] A 19.9-L tank is filled with H2 to a pressure of 200. atm. How many balloons (each 2.00 L) can be inflated to a pressure of 1.00 atm from the tank? Assume the ideal gas behavior, that there is no temperature change, and that the tank cannot be emptied below 1.00 atm pressure. balloon(s) Submit Answer Try Another Version 3 item attempts remaining Previous Next Email Instructor Save and Exitarrow_forwardCan you help me with thisarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY