
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
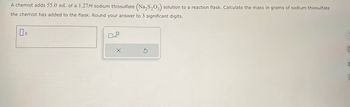
Transcribed Image Text:A chemist adds 55.0 mL of a 1.27M sodium thiosulfate (Na₂S₂O3) solution to a reaction flask. Calculate the mass in grams of sodium thiosulfate
the chemist has added to the flask. Round your answer to 3 significant digits.
X
S
6
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3 Consider the reaction 1205(g) + 5 CO(g) →→ 5CO2(g) + ¹2(g) 80.0 grams of iodine pentoxide, 1205 (molar mass = 333.80g/mol), reacts with 38.0 grams of carbon monoxide (molar mass 28.01g/mol). Determine the mass of iodine that could be produced. Place only the numeric answer in the box, no units/% sign. If the answer is 12.34g O2, only type 12.34 in the box. Round all answers to 2 decimal places Narrow_forwardCalculate the mass (in grams) of oxalic acid (H2C2O4) needed to make 100.0 mL of a 0.250 M solution. Please report your answer with the correct number of significant digits.arrow_forwardSuppose 5.25 g of nickel(II) bromide is dissolved in 100. mL of a 0.20 M aqueous solution of potassium carbonate. Calculate the final molarity of nickel(II) cation in the solution. You can assume the volume of the solution doesn't change when the nickel(II) bromide is dissolved in it. Be sure your answer has the correct number of significant digits.arrow_forward
- 410.0 mL of a 4.8 mol/L silver nitrate (AgNO 3 ) a reaction flask. Calculate the mass in kilograms of silver nitrate.arrow_forwardA chemist adds 110.0 of a 1.63 molcalcium bromide (CaBr 2 ) solution to a reaction flask Calculate the mass in grams of calcium bromide the chemist has added to the flask. Be sure your answer has the correct number of significant digits.arrow_forwardA chemist prepares a solution of vanadium(III) bromide (VBr) by measuring out 0.10 g of VBr3 into a 100. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Br anions in the chemist's solution. Be sure your answer is rounded to 2 significant digits. mol L x10 X Śarrow_forward
- How many grams of oxalic acid, H2C2O4, are required to prepare 750. mL of a 0.250 M oxalic acid solution? Report your result in decimal notation and to the proper number of significant figures.arrow_forwardA chemist prepares a solution of iron III bromide FeBr3 by measuring out 78.8mg of FeBr3 into a 100.mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Br− anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits.arrow_forwardA chemist prepares a solution of iron (II) chloride (FeCl₂) by measuring out 1.0 g of FeCl₂ into a 250. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Canions in the chemist's solution. Be sure your answer is rounded to 2 significant digits. 7 mol L 0x x10 X Śarrow_forward
- A chemist prepares a solution of vanadiumIII bromide VBr3 by measuring out 0.50g of VBr3 into a 50.mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Br− anions in the chemist's solution. Be sure your answer is rounded to 2 significant digits.arrow_forwardSuppose 1.24 g of sodium chloride is dissolved in 200. mL of a 28.0 m M aqueous solution of silver nitrate. Calculate the final molarity of sodium cation in the solution. You can assume the volume of the solution doesn't change when the sodium chloride is dissolved in it. Be sure your answer has the correct number of significant digits. ol M x10 Ararrow_forwardBalance the following chemical reaction. Enter the sum of the balanced coefficients as your answer. Assign "blank" coefficients a value of 1. silver iodide + sodium sulfide → silver sul fide + sodium iodidearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY