
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
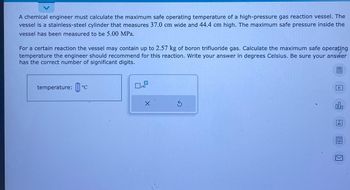
Transcribed Image Text:A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The
vessel is a stainless-steel cylinder that measures 37.0 cm wide and 44.4 cm high. The maximum safe pressure inside the
vessel has been measured to be 5.00 MPa.
For a certain reaction the vessel may contain up to 2.57 kg of boron trifluoride gas. Calculate the maximum safe operating
temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Be sure your answer
has the correct number of significant digits.
temperature: ºc
x10
X
S
olo
18
Ar
18
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 23.0 cm wide and 27.6 cm high. The maximum safe pressure inside the vessel has been measured to be 1.60 MPa. For a certain reaction the vessel may contain up to 0.239 kg of boron trifluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Be sure your answer has the correct number of significant digits. temperature: C 0 x10 × Ś ? 18 Ararrow_forwardA chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 43.0 cm wide and 51.6 cm high. The maximum safe pressure inside the vessel has been measured to be 8.30 MPa. For a certain reaction the vessel may contain up to 15.7 kg of chlorine pentafluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Be sure your answer has the correct number of significant digits. temperature: °C ☐ 10 Xarrow_forwardConsider the equilibrium system described by the chemical reaction below. Calculate the value of Qc for the initial set reaction conditions in 2.50 L container: 11.1 g C, 2.45 g H:O, 15.3 g CO and 7.55 g H2. C(s) + H:O(g) = CO(g) + Ha(g) Based on the given data, set up the expression for Qc and then evaluate it. Do not combine or simplify terms. Qc =arrow_forward
- The white powder of LiHCO3 is unstable and when heated the following reaction occurs: 2LiHCO3(s) → Li2CO3(s) + H2O(g) + CO2(g) A student performed a controlled experiment in which he heated 2.3 grams of LiHCO3 powder and collected the gases into a 1.4-liter container. At the end of the reaction the temperature in the tank was 350 ° C 1.a. Calculate how many grams of Li2CO3 were obtained at the end of the reaction. 1. b. Calculate the tank pressure at the end of the reaction. 1.c. Calculate the partial pressure of CO2 in the tank at the end of the reaction. 2. Answer the following questions for the reactants: CO2, H2O, Li2CO3, LiHCO3 a. Determine which of them has the weakest intermolecular forces. b. Determine which of the substances is a molecular substance with a constant dipole. c. Determine the systematic name for the ionic substances.arrow_forwardConsider the equilibrium system described by the chemical reaction below. Calculate the value of Qc for the initial set reaction conditions in a 2.00 L container: 0.0560 g H2, 4.36 g l2, and 1.32 g HI. H:(g) + lz(g) = 2 HI(g) Based on the given data, set up the expression for Qc and then evaluate it. Do not combine or simplify terms. Qc =arrow_forwardA sample of phosgene is sealed in a 250.0-cm³ glass bulb to which a pressure gauge is attached. The bulb is heated to 600 °C, and the gauge shows that the pressure in the bulb rises to 0.973 atm. At this temperature, the COCI₂(g) is partially dissociated into CO(g) and Cl₂(9) according to the equation CoCl₂(g) CO(g) + Cl₂(9) At 600 °C, Kp = 5.00 for this reaction. Assume that the contents of the bulb are at equilibrium and calculate the partial pressure the three different chemical species in the vessel. Pcocl₂ = Pco= Pc₂" atm atm atmarrow_forward
- Consider the following reversible reaction at equilibrium: C6H12O6(aq) + 6 O2(g) 6 CO2(g) + 6 H2O(l). Given that this reaction is exothermic, if heat is added to the equilibrium system, how is the stress relieved?arrow_forwardThe following system is at equilibrium in a closed vessel: 4 HCl(aq) + MnO2(s) ⇆ MnCl2(aq) + 2 Cl2(g) + 2 H2O(l) Various stresses are applied to the system as illustrated in the chart. Drag the appropriate label from the list below that indicates how the system responds to each stress. Each label can be used more than once.arrow_forwardWhile ethanol (CH,CH,OH) is produced naturally by fermentation, e.g. in beer- and wine-making, industrially it is synthesized by reacting ethylene (CH,CH,) with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 50 L tank with 15. mol of ethylene gas and 22. mol of water vapor. When the mixture has come to equilibrium he determines that it contains 7.8 mol of ethylene gas and 14.8 mol of water vapor. The engineer then adds another 5.5 mol of water, and allows the mixture to come to equilibrium again. Calculate the moles of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits. |molarrow_forward
- 1 H,(9) + 2 NO(g) · N,O(g) + H,O(g) k1 2 H2(g) + N,0(g) → N,(9) + H,O(g) k2 Suppose also k, «k,. That is, the first step is much slower than the second. Write the balanced chemical equation for the overall chemical reaction: 2H, (g) + 2NO(g) 2H,0(g) + N, Write the experimentally- observable rate law for the overall chemical reaction. rate = k H,NO2 Note: your answer should not contain the concentrations of intermediates. any Express the rate constant k for the overall chemical reaction in terms of k1, k2, and (if necessary) the rate constants k1 and k-2 for = K,] 21 the reverse of the two elementary reactions in the mechanism.arrow_forwardExplain your reasoning, please. Thank you.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY