
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
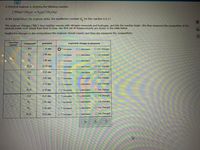
Transcribed Image Text:A chemical engineer is studying the following reaction:
2 NO(g)+2H,(g) – N(a)+2H,0(g)
At the temperature the engineer picks, the equilibrium constant K, for this reaction is 0.17.
The engineer charges ("fills") four reaction vessels with nitrogen monoxide and hydrogen, and lets the reaction begin. She then measures the composition of the
mixture inside each vessel from time to time. Her first set of measurements are shown in the table below.
db
Predict the changes in the compositions the engineer should expect next time she measures the compositions.
Ar
reaction
vessel
compound
pressure
expected change in pressure
NO
7.34 atm
Ot increase
OI decrease
O (no change)
H,
1.96 atm
O f increase
OI decrease
O (no change)
N
1.66 atm
O t increase
OI decrease
O (no change)
H,0
10.20 atm
O f increase
OI decrease
O (no change)
NO
9.18 atm
O t increase
OI decrease
O (no change)
H.
3.80 atm
O f increase
OI decrease
O (no change)
B
N,
0.74 atm
O f increase
OI decrease
O (no change)
HO
8.36 atm
O f increase
OI decrease
O (no change)
NO
8.30 atm
O increase
O! decrease
O (no change)
H.
2.92 atm
O f increase
O ! decrease
O (no change)
N
OI decrease
O (no change)
1.18 atm
O t increase
H,O
924 atm
O f increase
OI decrease
O (no change)
8 o o oo
O o
O o
目县|| 目具
|品S さ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Carbon monoxide is a colorless, odorless gas that binds irreversibly to hemoglobin in our blood, causing suffocation and death. Co is formed during incomplete combustion of carbon. One way to represent this equilibrium is: 2c0(g)=2 C(s) + 02(g) We could also write this reaction three other ways, listed below. The equilibrium constants for all of the reactions are related. Write the equilibrium constant for each new reaction in terms of K, the equilibrium constant for the reaction above. 1) C(s) + 1/2 02(g)=co(g) K = 2) 2 C(s) + 02(g) =2c0(g) K2 = %3D 3) CO(g) C(s) + 1/2 02(g) K3 = Drag and drop your selection from the following list to complete the answer: 1/K K1/2 (1/K)1/2arrow_forwardA chemical engineer is studying the following reaction: HCN(aq)+NH,(aq) → CN (aq)+NH(aq) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 1.0. The engineer charges ("fills") four reaction vessels with hydrogen cyanide and ammonia, and lets the reaction begin. He then measures the composition of the mixture inside each vessel from time to time. His first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time he measures the compositions. reaction compound concentration expected change in concentration vessel HCN 0.06 M O f increase OI decrease O (no change) NH, 0.22 M f increase OI decrease (no change) A CN 0.97 M increase I decrease (no change) NH, 1.23 M f increase OI decrease O (no change) HCN 0.53 M f increase OI decrease (no change) NH, 0.69 M f increase O I decrease (no change) В CN 0.50 M t increase OI decrease O (no change) NH, 0.76 M f increase Ot decrease (no change)…arrow_forwardchemical engineer is studying the following reaction: BF3(aq)+NH3(aq) → BF3NH3(aq) At the temperature the engineer picks, the equilibrium constant K for this reaction is 0.61. The engineer charges ("fills") three reaction vessels with boron trifluoride and ammonia, and lets the reaction begin. He then measures the composition of the mixture inside each vessel from time to time. His first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time he measures the compositions. reaction vessel compound concentration expected change in concentration A BF 3 NH₂ 0.98 M Of increase ↓ decrease O(no change) 0.84 M O↑ increase Odecrease BF, NH₂ 0.50 M O↑ increase O decrease (no change) O(no change) BF3 1.42 M O increase ↓ decrease O(no change) B NH3 1.28 M O↑ increase ↓ decrease O(no change) BF3NH3 0.06 M O↑ increase Odecrease (no change) BF3 0.54 M O↑ increase Odecrease C NH₂ 0.40 M ↑ increase BF3NH3 0.94 M O↑ increase O↓…arrow_forward
- Sulfur dioxide and oxygen react to form sulfur trioxide, like this: 2 SO₂(g) + O₂(g) → 2 SO 3(g) The reaction is exothermic. Suppose a mixture of SO2, O₂ and SO3 has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation The temperature is lowered. change in composition The pressure of SO3 will The temperature is raised. The pressure of SO₂ will ? ? î ↑ shift in equilibrium O X to the right to the left (none) to the right to the left (none) Sarrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 CH₂(g) C₂H₂(g) + 3H₂(g) K₂=6. × 10 -8 He fills a reaction vessel at this temperature with 7.0 atm of methane gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of C₂H₂, using only the tools available to you within ALEKS? If you said yes, then enter the equilibrium pressure of C₂H₂ at right. Round your answer to 1 significant digit. 0 yes no atm x10 X Śarrow_forwardHydrogen and fluorine react to form hydrogen fluoride, like this: H,(g)+F2(g) → 2 HF (g) The reaction is exothermic. Suppose a mixture of H,, F, and HF has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. 圖 do perturbation change in composition shift in equilibrium O to the right The temperature is raised. The pressure of H2 will O to the left go up. (none) go down. not change. to the right The temperature is lowered. to the left The pressure of HF will O (none) Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use I Privacy Center Accessibility DII 80 F10 FB F9 F6 F7 FS F4 esc F3 F1 @ # $ dele - 7 8. 2 3 4arrow_forward
- Carbon dioxide and water react to form methanol and oxygen, like this: 2 CO,(9)+4 H,0(g)→2 CH;OH(1)+3 O2(9) At a certain temperature, a chemist finds that a 7.2 L reaction vessel containing a mixture of carbon dioxide, water, methanol, and oxygen at equilibrium has the following composition: compound amount CO, 3.88 g H,0 4.38 g CH;OH 4.22 g O2 1.08 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K_ = 0arrow_forwardAt a certain temperature, the equilibrium constant K for the following reaction is 3.44 x 10°: NO, (g) + CO(g)= NO(g) + CO,(g) Use this information to complete the following table. Suppose a 50. L reaction vessel is filled with 0.81 mol of NO2 and 0.81 mol of CO. What can you say about the composition of the mixture in the vessel at equilibrium? O There will be very little NO2 and CO. x10 There will be very little NO and CO2. ? Neither of the above is true. What is the equilibrium constant for the following reaction? Round your answer to 3 significant digits. K =0 NO(g)+CO2(9) NO2(9)+CO(9) What is the equilibrium constant for the following reaction? Round your answer to 3 significant digits. = 0 K = 2 NO,(9)+2CO(9) 2 NO(9)+2CO2(9)arrow_forwardCarbon disulfide and oxygen react to form carbon dioxide and sulfur dioxide, like this: cs,(e)+30,(2) → CO,(2)+2S0,(g) Suppose a mixture of CS,, O,, CO, and SO, has come to equilibrium in a closed reaction vessel. Predict what change, if any, the pertur below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation change in composition shift in equilibrium Oto the right The pressure of O, will Some CS, is removed. O to the left The pressure of CO, will O (none) O to the right Some Co, is added. The pressure of CS, will O to the left The pressure of O, will O (none) Explanation Check P Type here to search O 2022 McGraw H LLC Al Rights Reserved Terms of Use Pivac 99+ ook Alt Ararrow_forward
- Sulfur dioxide and oxygen react to form sulfur trioxide, like this: 2 SO,(9)+O2(g) → 2 SO3(g) The reaction is exothermic. Suppose a mixture of SO2, O, and SO, has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. dlo perturbation change in composition shift in equilibrium Ar to the right The temperature is lowered. to the left The pressure of SO3 will ? (none) to the right to the left The temperature is raised. The pressure of SO2 will ? O (none)arrow_forwardA chemical engineer is studying the following reaction: HCH3CO2(aq)+CH3NH2(aq) → CH3CO2(aq)+CH3NH(aq) At the temperature the engineer picks, the equilibrium constant K for this reaction is 0.80. The engineer charges ("fills") four reaction vessels with acetic acid and methylamine, and lets the reaction begin. She then measures the composition of the mixture inside each vessel from time to time. Her first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time she measures the compositions. reaction vessel compound concentration expected change in concentration HCH CO₂ 0.58 M ↑ increase ↓ decrease (no change) CH3NH2 0.57 M ↑ increase ↓ decrease (no change) A CH3CO2 1.09 M ○ ↑ increase ↓ decrease (no change) CH,NH, 1.28 M ○ ↑ increase ↓ decrease (no change) HCH,CO₂ CH3NH2 1.28 M ↑ increase ↓decrease (no change) 1.27 M ↑ increase ↓ decrease (no change) B CH,CO, 0.39 M ↑ increase ↓ decrease (no change) CHÍNH, HCH3CO2…arrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 CH₂(g) C₂H₂(g) + 3H₂(g) K₁=3. × 10 P -6 He fills a reaction vessel at this temperature with 6.0 atm of methane gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of C₂H₂, using only the tools available to you within ALEKS? If you said yes, then enter the equilibrium pressure of C₂H₂ at right. Round your answer to 1 significant digit. 0 yes no atm x10 X Śarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY