
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
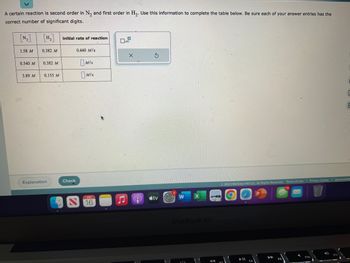
Transcribed Image Text:A certain reaction is second order in N₂ and first order in H₂. Use this information to complete the table below. Be sure each of your answer entries has the
correct number of significant digits.
1.58 M
0.540 M
3.89 M
0.382 M
0.382 M
0.155 M
Explanation
initial rate of reaction
Check
0.440 M/s
Mis
MIS
X
S
tv
W
MacBook Air
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center
C
5
Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A certain reaction is second order in N₂ and second order in H₂. Use this information to complete the table below. Round each of your answers to 3 significant digits. [¹₂] 1.57 M 1.57 M 2.50 M [H₂] 0.696 M 2.10 M 0.437 M initial rate of reaction 8.00 × 10 M/S M/S M/s 0 ☐x10 Xarrow_forwardThe initial concentration of reactant in a first order reaction is 0.27 M. The rate constant for the reaction is 0.75 s-1. What is the concentration (in mol/L) of reactant after 1.5 seconds? 3.8 1.7 0.088 0.020arrow_forwardA certain reaction is second order in N₂ and first order in H₂. Use this information to complete the table below. Be sure each of your answer entries has the correct number of significant digits. [N₂] 1.85 M 4.28 M 7.27 M [H₂] 0.976 M 0.976 M 0.248 M initial rate of reaction 3 4.00 X 10 M/s M/s M/S x10 × Śarrow_forward
- A certain reaction is first order in N₂ and second order in H₂. Use this information to complete the table below. Round each of your answers to 3 significant digits. [₂] [H₂] initial rate of reaction 1.05 M 3.79 M 0.358 M 0.544 M 0.544 M 1.60 M 79.0 M/s M/S M/S 0 x10 Xarrow_forwardA certain reaction is second order in N₂ and first order in H₂. Use this information to complete the table below. Round each of your answers to 3 significant digits. [₂] 1.06 M 2.78 M 0.431 M [H₂] initial rate of reaction 0.782 M 0.782 M 1.93 M 0.0378 M/s M/s M/S Xarrow_forwardA certain reaction is second order in N₂ and second order in H₂. Use this information to complete the table below. Round each of your answers to 3 significant digits. 0.460 M 0.460 M 1.43 M [¹2₂] 1.66 M 2.94 M 0.534 M initial rate of reaction 56.0 M/s M/S M/S 0 x10 Xarrow_forward
- A certain reaction is second order in N, and first order in H₂. Use this information to complete the table below. Round each of your answers to 3 significant digits. [₂] 1.94 M 1.94 M 3.73 M 1.34 M 6.33 M 0.697 M initial rate of reaction 0.349 M/s M/S M/S Xarrow_forwardA certain reaction is first order in H₂ and first order in I₂. Use this information to complete the table below. Round each of your answers to 3 significant digits. 2.31 M 1.10 M 4.73 M [¹2] 1.53 M 1.53 M 0.747 M initial rate of reaction 2.00 x 10 M/s MIS MIS O x10 X 5arrow_forwardA certain reaction is first order in H₂ and first order in I₂. Use this information to complete the table below. Round each of your answers to 3 significant digits. [H₂] 1.53 M 0.308 M 0.502 M [4₂] 0.663 M 0.663 M 2.02 M initial rate of reaction 10³ 2.00 x 10 M/s Mis M/s olo Ärarrow_forward
- A certain reaction is first order in H₂ and first order in I₂. Use this information to complete the table below. Round each of your answers to 3 significant digits. [¹₂] [¹₂] initial rate of reaction 1.99 M 0.683 M 5.00 M/s 1.99 M 2.54 M MIS 5.71 M 0.238 M M/S olo Ararrow_forwardThe following data was collected for the reaction 3 H2 + N2 2 NH3. Time (s) [H2] (M) 1.00 10 0.75 Based on these data, what is the average rate of the reaction in the time period from 0 s to 10 s? O 0.0125 M/s 0.025 M/s O -0.025 M/s 0.050 M/s 0.00833 M/s 0.075 M/sarrow_forwardI need help with both of the blank spaces with correct significant figuresarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY