
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Using the Kf and Kb equations with electrolytes
Transcribed Image Text:-1
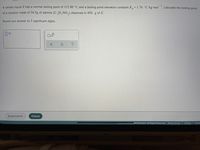
A certain liquid X has a normal boiling point of 115.80 °C and a boiling point elevation constant K,= 1.76 °C-kg mol . Calculate the boiling point
%3D
of a solution made of 34.5g of alanine (C,H,NO,) dissolved in 450. g of X.
Round you answer to 5 significant digits.
x10
Explanation
Check
O 2020 MCOiaw-Hill Education. All Rights Reserved. Terms of Use Privacy I Acce:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Titration of a 86.00mL KOH solution required 398.00 ml of 0.0240 M acetic acid. What is the molarity of KOH solution?arrow_forwardAn aqueous solution contains 0.28 M hydrofluoric acid. One Liter of this solution could be converted into a buffer by the addition of: (Assume that the volume remains constant as each substance is added.) (Select all that apply.) 0.14 mol HBr0.28 mol NaI0.29 mol NaF0.14 mol KOH0.29 mol HBr An aqueous solution contains 0.28 M ammonia. One liter of this solution could be converted into a buffer by the addition of: (Assume that the volume remains constant as each substance is added.) 0.29 mol NH4ClO4 0.14 mol HBr 0.29 mol HBr 0.14 mol Ca(OH)2 0.28 mol Ba(ClO4)2arrow_forwardPlease calculate the pOH for a 6.65 M solution of a weak base, X with K = 2.77 x 10-4. Please express your answer to in decimal notation with appropriate sig fig. Type your answer...arrow_forward
- A solution is made by adding HCN to yield a concentration of 0.01 M and KCN to yield a concentration of 0.01 M. Using the algebraic approximation method, (a) What is the pH of the solution if activity corrections are ignored? (b) What is the pH of the solution if activity corrections are included?arrow_forwardsomeone please help me with these questions! ?arrow_forwardwhat is the balance equation wirh physical sates of butanoic acid and potassium butanoate as buffer solution.arrow_forward
- If 17.1 mL of 0 M NaOH are used, how many moles are in this sample? Suppose 0.371 grams of a solid monoprotic acid are titrated with 16.45 mL of 0.117 M NaOH. What is the molar mass of the solid acid?arrow_forwardThe maximum amount of calcium sulfite that will dissolve in a 0.149 M ammonium sulfite solution is ?M.arrow_forwardIt was assumed: a) Before any acetic acid was added, essentially all the indicator was in the In- form. b) After the HCl was added, essentially all the indicator was in the HIn form. Justify these assumptions using the measured pH of these solutions and your experimental value for pKHIn to determine: 1) The fraction fo the indicator in the HIn form in solution B before any of solution A was added 2) The fraction of the indicator in the In- form after the HCl was added.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY