
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
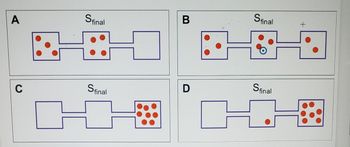
Transcribed Image Text:A
с
final
Sfinal
1
B
D
final
H
Sfinal
H
+
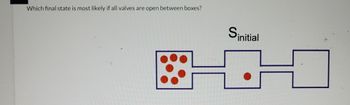
Transcribed Image Text:Which final state is most likely if all valves are open between boxes?
initial
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Eto OEt 1. NaOCH3 2. H*, H₂O, heat 22 Marrow_forwardmart-Hiring Ce... F3 # E D C F4 4 AH for an endothermic process is process is R F s F5 V 5 F6 A) positive, zero G B) negative, positive C) positive, negative D) zero, negative TJL F7 B Y H Question 1 of 5 F8 & 7 N DELL F9 J * 8 1 M F10 ( K while AH for an exothermic F11 ) O L F12 P PrtScr ( 1 w@D 4x Insert Delete Backspacearrow_forwardSee Figure 10-2. What is the reactant that would yield the two products shown as major products? OA OB C ODarrow_forward
- 1. obtain thenuctrition facts infarmodionfor Cheeatos, andrecord the numberof Calaries per servinqandthe number of qrams per servinq. Calculote the number of Calories.containedlin one gana af Cheetos Caleries per Servu Lng leo + f ৭৮om per ০০n 289arrow_forward9. HO OH cat. H₂SO4 ∞arrow_forwardGiven the data in the table below, AHO for the reaction is rxn Report your answer to one decimal place and with the appropriate sign. 3A₂B3 + CB 2A3B4+ CB2 A₂B3 A3B4 CB CB₂ Substance -> AHOrxn (kJ) -662.9 -285.4 -189.8 -465.7 27 kJ.arrow_forward
- 5 The specific heats of aluminum and iron are 0.214 and 0.107 calories per gram degrees Celsius [cal/g °C)] respectively. If we add the same amount of energy to a cube of each material (of the same mass) and find that the temperature of the aluminum increases by 30 degrees Fahrenheit [F], how much will the iron temperature increase in degrees Fahrenheit [F]? esc 27 Click the icon to view conversion formulas for temperature scales. 28 Click the icon to view the factors for conversions involving temperature differences. 29 Click the icon to view the conversion table. 30 Click the icon to view the table of common derived units in the Sl system. The iron temperature will increase by 27: More Info 28: More Info 1 29: More Info @ 2 # # 3 Q $ 4 °F. (Round your answer to the nearest whole number.) % T[ F]-32 T[°C] -0 180 100 T[K] = T[°C]+273 T[ R] = T[°F] + 460 Mass 1 kg = 2.205 lbm 1 slug = 14.5939 kg 5 Temperature Change 1 K = 1 °C = 6 = 1.8 °F = 1.8 °R Energy 1 J = 0.239 cal = 9.48 x 10-4…arrow_forward3. Predict the major products of the following reactions. Include stereochemistry where appropriate.arrow_forwardN2(g) + O2(g)< -----> 2 NO(g) H = +180.7 kJ 2 H2O(l) <-----> 2 H2(g) + O2(g) H = +571.7 kJ 4 NH3(g) + 5 O2(g) <-----> 4 NO(g) + 6 H2O(l) H = -1167.1 kJ Use Hess's law to calculate H for the reaction below. 2 NH3(g) <-----> N2(g) + 3 H2(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY