
Advanced Engineering Mathematics
10th Edition
ISBN: 9780470458365
Author: Erwin Kreyszig
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Question
A) Determine the mass of salt in the tank after t min
B) When will the concentration of salt in the tank reach 0.02 kg/L?
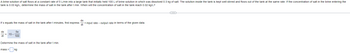
Transcribed Image Text:A brine solution of salt flows at a constant rate of 5 L/min into a large tank that initially held 100 L of brine solution in which was dissolved 0.3 kg of salt. The solution inside the tank is kept well stirred and flows out of the tank at the same rate. If the concentration of salt in the brine entering the
tank is 0.03 kg/L, determine the mass of salt in the tank after t min. When will the concentration of salt in the tank reach 0.02 kg/L?
dx
If x equals the mass of salt in the tank after t minutes, first expressat input rate-output rate in terms of the given data.
dx
dt
= 15-
5x
100
Determine the mass of salt in the tank after t min.
mass= kg
=
←
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- e) A spring is stretched by 6 % to 34 cm. The length before the stretch was cm (f) The volume of liquid in a barrel decreases by 21 % to 8.8 litres after some liquid leaked out. The original volume was litres.arrow_forwardWater is flowing at the rate of 3 km/hr theough a circular pipe of 20 cm internao diameter into a circular cistern of diameter 10 m and depth 2m.How much time will the cistern be filled ?arrow_forwardPlease answer the question and show solution, i will rate you immediately.arrow_forward
- H.W. 1) Calculate the volume of cylinder has 15 m height and 8 m diameter. 2) If we have other cylinder with the same height but it volume greater than first cylinder by 20%, how long its radius? 3) Write a program to calculate the volumetric and mass flow rate of a liquid flowing in a pipe with a velocity equal to 0.5 m/s. Knowing that the diameter of this pipe is 0.1 m and the density of this liquid is 890 kg/m³ ? 4) For the following distillation column write a code to find the value of stream B and the compositions of stream D? D=80 X% ? S%? T%? Z% ? F=100kg 15% X 25% S 40% T 20% Z B=? 15% X 25% S 40% T 20% Z 9 5) Compute the reaction rate constant for a first-order reaction given by the -E/RT Arrhenius law k = A e at a temperature T = 500 K. Here the activation energy is E=20 kcal/mol and the pre-exponential factor is A=10¹3 s¹. The ideal gas constant is R=1.987 cal/mol K.arrow_forwardThe ammonia solution that is purchased for a stockroom has a molarity of 15 M. De- termine the volume of 15 M NH3(aq) that must be diluted to 500 mL to prepare 1.75 M NH3(aq). Answer in units of mL.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Advanced Engineering MathematicsAdvanced MathISBN:9780470458365Author:Erwin KreyszigPublisher:Wiley, John & Sons, Incorporated
Advanced Engineering MathematicsAdvanced MathISBN:9780470458365Author:Erwin KreyszigPublisher:Wiley, John & Sons, Incorporated Numerical Methods for EngineersAdvanced MathISBN:9780073397924Author:Steven C. Chapra Dr., Raymond P. CanalePublisher:McGraw-Hill Education
Numerical Methods for EngineersAdvanced MathISBN:9780073397924Author:Steven C. Chapra Dr., Raymond P. CanalePublisher:McGraw-Hill Education Introductory Mathematics for Engineering Applicat...Advanced MathISBN:9781118141809Author:Nathan KlingbeilPublisher:WILEY
Introductory Mathematics for Engineering Applicat...Advanced MathISBN:9781118141809Author:Nathan KlingbeilPublisher:WILEY Mathematics For Machine TechnologyAdvanced MathISBN:9781337798310Author:Peterson, John.Publisher:Cengage Learning,
Mathematics For Machine TechnologyAdvanced MathISBN:9781337798310Author:Peterson, John.Publisher:Cengage Learning,


Advanced Engineering Mathematics
Advanced Math
ISBN:9780470458365
Author:Erwin Kreyszig
Publisher:Wiley, John & Sons, Incorporated

Numerical Methods for Engineers
Advanced Math
ISBN:9780073397924
Author:Steven C. Chapra Dr., Raymond P. Canale
Publisher:McGraw-Hill Education

Introductory Mathematics for Engineering Applicat...
Advanced Math
ISBN:9781118141809
Author:Nathan Klingbeil
Publisher:WILEY

Mathematics For Machine Technology
Advanced Math
ISBN:9781337798310
Author:Peterson, John.
Publisher:Cengage Learning,

