
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
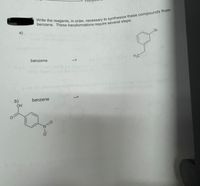
Transcribed Image Text:a)
Br
benzene
H3C
-->
benzene
b)
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw these molecules 1. CH;CH,CH,CH,CH 2 CH;CH,ČCH,CH3 N .arrow_forwardIdentify whether each compound is a hydrocarbon or another organic compound. Drag the items into the appropriate bins. Reset Help H3C-CH || H3C- CH, – C- CH2 – CH3 H;CO– CH2 – CH3 || H3C- CH3 Hydrocarbon Other organic compoundarrow_forward2. Identify the functional group in each molecule shown below A) CH₂-OH | CH-OH B) CH₂-OH omework 1 CH,NHCH2CH3 D) O || C) CH3-C-0—CH2-CH2-CH3arrow_forward
- 15) Name the structure. A) 4-chlorocyclohexene B) 1-chloro-3-cyclohexene C) 4-chlorocycloheptane D) 4-chlorocycloheptene E) 1-chloro-3-cycloheptenearrow_forwardN|ON|OU|G fic app.101edu.co Maps YouTube 1 N L H3C-CH₂ H3C Q A N G A © 2 - F2 W S C= X H Gb| CCC |2bA|QQ Question 7 of 31 The molecule shown here is classified as what type of organic compound? A) alkane CH3 B) alkene C) alkyne CH₂-CH3 D) aromatic compound E) aldehyde V Aa v > #3 II > 80 F3 E D C $ 4 Q F4 R F D, 67 dº % 5 V F5 T BAX G d G MacBook Air F6 B Y & 7 H F7 U N * 8 J DII F8 M ( 9 K S|G1|CS UONEE F9 O ) H L 7 F10 Done P F11 B ← L x Gra + 11 KR ☐ F12 +arrow_forwardHow can you tell, from its chemical formula, if a hydrocarbon is an alkane, on one hand, or if there is a ring or pi bond on the other. Thank you.arrow_forward
- For part b. Identify as an Alkane, alkene, alkyne, aromatic, alcohol, ether, aldehyde, ketone, carbocylic acid, Ester, or amine.arrow_forward6) Combustion of hydrocarbons: C4H8+ 6 0₂ -->arrow_forwardSafari File Edit View History Bookmarks Window Help 36% O A АBС Thu 3:08 PM 5. The general formula of alkene is C„H2n. (a) The relative molecular mass of an alkene X is 70.0 . Find out the molecular formula of X. (Relative atomic masses: C = 12.0, H = 1.0) (b) X is a straight chain alkene showing cis-trans isomerism. (i) Give the structural formula of X. (ii) Draw a 3-dimension diagram of the trans-isomer of X.arrow_forward
- Naming branched alkanes Name the following organic compounds: compound CH₁₂ | - - CH₂ — CH₂▬ CH₂ | CH - name CH3 血 - - - CH2 CH2 CH2 - - CH3 CH2 — CH₂ — C - - - - CH₂ — CH₂ — CH3 CH, CH₂ CH3 - CH | CH₂ - CH2- - П CH2-CH3 ☐arrow_forwardCO Ch app.101edu.co be Maps SC Bb Bl ! 1 H3C-CH₂ H3C F1 Q A 1 option CH N @ 2 2 Ac Be G dc B H [S Question 37 of 48 The molecule shown here is classified as what type of organic compound? A) alkane B) alkene CH3 C=C C) alkyne D) aromatic compound E) aldehyde DII F8 F2 T W S X дв command #3 80 F3 E D CH₂-CH3 F4 с $ 4 R F % 5 V F5 T X G 6 B F6 Y H & 7 F7 U N 8 J w Mb Hc G 17 CS UO NE Et | | M ( 9 K F9 O H ) O L F10 P F11 WE { + 11 [ t command option Ba Gra C F12 } 1 + dearrow_forwardWhat is the relationship, if any, between these organic compounds?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY