
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
i need help i got the partial pressures wrong and need help please
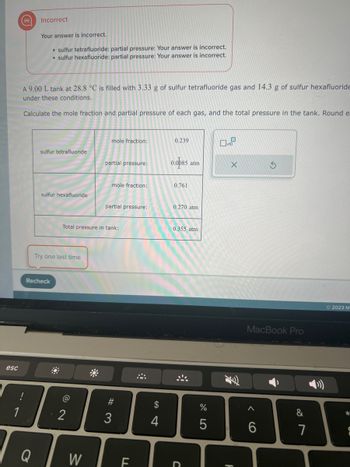
Transcribed Image Text:The task involves calculating the mole fraction and partial pressure of sulfur tetrafluoride and sulfur hexafluoride gases in a 9.00 L tank at 28.8 °C.
**Initial Problem Statement:**
- A 9.00 L tank is filled with 3.33 g of sulfur tetrafluoride gas and 14.3 g of sulfur hexafluoride gas.
- The task is to calculate the mole fraction and partial pressure of each gas and the total pressure in the tank.
**Results from the Table:**
1. **Sulfur Tetrafluoride:**
- **Mole fraction:** 0.239
- **Partial pressure:** 0.085 atm
2. **Sulfur Hexafluoride:**
- **Mole fraction:** 0.761
- **Partial pressure:** 0.270 atm
3. **Total pressure in tank:**
- 0.355 atm
**Feedback Area:**
- Incorrect answers are noted for both gases' partial pressures.
**Instructions:**
- A prompt to "Try one last time" is displayed, along with a "Recheck" button to submit revised answers.
This exercise is an example of applying gas laws to real-world problems, specifically focusing on computing mole fractions and partial pressures of gas mixtures under specific conditions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The density of __________ is 0.900 g/L at STP. A) CH4 B) Ne C) CO D) N2 E) NO *please write down steps!*arrow_forwardQuestion 40 48.5 mL of an ideal gas is in a syringe with a pressure of 102.5 kPa. What will the pressure inside the syringe be if the gas is compressed to 17.5 mL if the temperature remains constant and the syringe is perfectly sealed? 284.1 kPa 37.0 kPa 332.6 kPa 206.6 kPaarrow_forwardIn the open ended manometer (open to the atmosphere) pictured below, what is the pressure of the gas in the bulb if the pressure of the atmosphere is 755 mm Hg and h = 55 mm Hg? Input your answer in mm Hg.arrow_forward
- What is the pressure due to just dry oxygen gas at 21.0 Celcius in a wet gas sample that has a total pressure of 751.8 mmHg? Use table 1. Show all work and circle your correctly rounded answer. Table 1. Temp: 17.0 18.0 19.0 20.0 21.0 22.0 23.0 24.0 25.0 26.0 P Water Vapor, mmHG 14.5 15.5 16.5 17.5 18.7 19.8 21.0 22.4 23.8 25.2arrow_forwardConsider the image of a closed-end manometer below. The fluid in the manometer is mercury. Calculate the pressure in the gas chamber. Gas Closed end 6.39 cmarrow_forwardAnswer both 1 &2 .if u answer only 1st quesion I will downvotearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY