
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Calculate the mole fraction from the information given below.
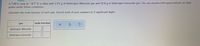
Transcribed Image Text:A 7.00 L tank at -8.7 °C is filled with 2.51 g of dinitrogen difluoride gas and 15.6 g of dinitrogen monoxide gas. You can assume both gases behave as ideal
gases under these conditions.
Calculate the mole fraction of each gas. Round each of your answers to 3 significant digits.
gas
mole fraction
dinitrogen difluoride
dinitrogen monoxide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the molecular gas density for an ideal gas at 400 K at a pressure of 10-9 Torr. (giving your answer in molecules m-³)arrow_forwardA gas with a volume of 0.56 L is initially at an unknown temperature. The gas is submerged in boiling water (100°C) and is determined to occupy a volume of 0.294 L at constant pressure and composition. What was the initial temperature of the gas in K?arrow_forwardBurning one mole of propane in a gas grill as 132 of carbon dioxide CO2 to the atmosphere. What volume in L of CO2 does this correspond to at STParrow_forward
- A 755 mL sample of gas which contains 0.0588 moles kept at 450. K would be under what pressure?arrow_forwardIf Ar with a partial pressure of 742.4 torr is mixed with He with a partial pressure of 6.06 torr, the total pressure according to your calculator would be 748.46 torr. The best answer, according to the rules for significant figures, would be:arrow_forwardIf pressure and temperature remain constant, the volume of a gas increases with increasing number of gas particles. True Falsearrow_forward
- Scientists agreed upon a standard temperature and pressure (STP) to study the properties of gases at to be consistent amongst their measurements. Temperature is C (or 273K) and pressure is 1.00 atm. Use the ideal gas law to determine the volume of one mole of any gas at STP (the molar volume).arrow_forwardA mixture of gases contain 2.01 g of element A and 2.01 g of element B. Element A has a molar mass of 12.1 g/mol. Element B has a molar mass of 52.1 g/mol. What is the mole fraction of element B? • Report with the correct number of significant figures. • Include the correct unit. Your Answer: Answer unitsarrow_forwardIn daltons and henrys law, why does the percentage of gasses in the air not decrease but the partial pressure does?arrow_forward
- A gas with a volume of 0.553 L is initially at an unknown temperature. The gas is submerged in boiling water (100oC) and is determined to occupy a volume of 0.247 L at constant pressure and composition. What was the initial temperature of the gas in K?arrow_forwardSolve correctly please.arrow_forwardi need the answer quicklyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY