
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Question 15.a of 15
Submit
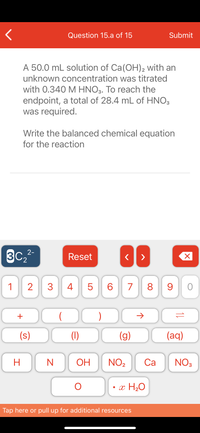
A 50.0 mL solution of Ca(OH)2 with an
unknown concentration was titrated
with 0.340 M HNO3. To reach the
endpoint, a total of 28.4 mL of HNO3
was required.
Write the balanced chemical equation
for the reaction
3C2
2-
Reset
1
2
3.
4
6.
7
8.
+
)
(s)
(1)
(g)
(aq)
N
OH
NO2
Са
NO3
• x H2O
Tap here or pull up for additional resources
LO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 185 mL of 0.355 M NaCl is added to a 0.500 L volumetric flask and the flask is filled with water. Calculate the [Na⁺] concentration in the diluted solution.arrow_forwardA 25.50 mL sample of calcium hydroxide is titrated with 0.010 M HCl and 2 drops of methyl orange indicator. At the end point of the titration (peach color persists) 7.60 mL of HCl has been added. What is the molar concentration of the hydroxide?arrow_forwardAn aqueous solution of Ca(OH)2with a concentration of 0.188 M was used to titrate 25.00 mL of aqueous HCl. 18.63 mL of the Ca(OH)2was required to reach the endpoint of the titration. How many moles of base were required to react completely with the acid in this reaction? How many moles of HCl were present in the original 25.00 mL of acid?arrow_forward
- A titration was done using 32.83mL of a weak acid and 0.141 M NaOH. If 32.81 mL of the NaOH was required to reach the equivalence point, what was the initial concentration of the weak acid?arrow_forwardA 10.00 mL sample of H2SO4 acid solution requires 22.53 mL of 0.1000 M NaOH for titration. What is the molarity of the acid solution?arrow_forwardA 25.00 mL aliquot is withdrawn from the liquid portion of saturated Manganese (II) hydroxide and transferred to an Erlenmeyer flask. After the addition of methyl orange, a titration with 0.0045 M HCl was performed. The endpoint was reached after 8.72 mL of the acid was added. What is the balanced chemical equation? How many moles of hydroxide ions were neutralized in the reaction? What is the molar concentration of hydroxide in the saturated solution? What is the molar concentration of Magnesium ion in solution?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY