
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Give typed explanation of all subparts not a single word hand written otherwise leave it
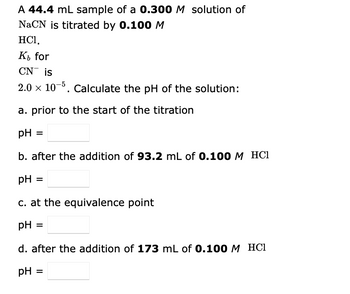
Transcribed Image Text:A 44.4 mL sample of a 0.300 M solution of
NaCN is titrated by 0.100 M
HC1,
K₁ for
CN is
2.0 × 10-5. Calculate the pH of the solution:
a. prior to the start of the titration
pH =
b. after the addition of 93.2 mL of 0.100 M HCl
pH =
c. at the equivalence point
pH:
d. after the addition of 173 mL of 0.100 M HCl
pH:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Here are the formulas for each substance: Sucrose: C12H22O11C_{12}H_{22}O_{11}C12H22O11 Copper (I) Chloride: CuClCuClCuCl Iodine: I2I_2I2 Potassium Iodide: KIKIKI Our goal is to try to find a pattern for which compounds conduct electricity when dissolved in water, and which don't. One hint is to look for patterns in which kinds of atoms form substances that conduct when dissolved, and which kinds of atoms form substances that don't conduct when dissolved. Here is a periodic table you can use. Let's start by finding the location on the periodic table for the atoms for substances that conduct when dissolved. What do you notice about where the atoms are?arrow_forwardThree samples (A, B, and C) of pure substance composed ofsodium, sulfur, and oxygen were isolated and purified. Elemental analyses showed that sample-A contained 1.62 g sodium, 1.13 g sulfur and 2.25 g oxygen; sample-B contains 1.60 g sodium,2.23 g sulfur, and 1.67 g oxygen; while sample-C contains 1.46 g sodium, 1.02 g sulfur, and 2.03 g oxygen. Determine whether A, B,and Care samples of the same or different compounds.arrow_forwardA heartburn tablet (like Tums or Rolaids) contains food grade calcium carbonate (s) to neutralize stomach acid, HCl(aq). Calcium carbonate is part of the rock cycle in the world of geology and is a type of sedimentary rock called limestone, that forms in oceans. The White Cliffs of Dover England and the Seven Sisters of Sussex England shown in the picture here are also made of calcium carbonate, specifically chalk. Chalk is a pure white limestone formed from the remains of tiny marine organisms (plankton) that lived and died in clear warm seas that covered much of Britain around 70 to 100 million years ago. Do not eat chalk to neutralize stomach acid as it is not purified food grade! Write the balanced molecular equation including phases for solid calcium carbonate reacting with hydrobromic acid. Then write the Full ionic equation including phases. Finally, write the net ionic equation including phases.arrow_forward
- 2. (i) State an example of a compound that is not a molecule and state an example of a molecule that is not a compound. Then, (ii) explain how you made your choices.arrow_forwardWrite a discussion on the topic of chemical identity by a conductivity test and melting point test?arrow_forwarddoes hydrogen exist as an atomic element in the air?arrow_forward
- n lo Report Form, Density of Nails, Baby Oll, ana MOPE Analysis of Results and Other Questions Densities (in g/cm at 25°C) 6. Assuming your nails are made of one of the elemental metals in the table (right), what is the most likely Mg 1.74 metal? How confident are you that they are made up of that metal? Explain. Zn 7.14 Cr 7.15 Sn 7.26 niloded lo sl Fe 7.87 Ni 8.90 8.96 Pb 11.3 Au 19.3 o dee 7. (a) Search the web for a value for the density of baby oil (if no T is given, it is assumed to be near room T): Source company or site name where you got the value (don't list the full URL): (b) Search the web (Google is best here) for the density of mineral oil from Sigma-Aldrich (a chemical company): (c) As you learned during your prelab (hopefully!), baby oil and mineral oil are not pure substances. As such, their composition (and thus density) can vary a bit, which would not be so for a pure substance (at a given T). That is why you likely found varying values-or ranges of values-for baby…arrow_forwardNonearrow_forwardQuestion 34 of 50 > 0.5 / 1 View POIICIES Show Attempt History Current Attempt in Progress Mercury, water, and bromine are liquids at standard temperature. Their molar entropies are in the sequence H2O < Hg < Br2. Incorrect. Using molecular properties, explain why bromine is more disordered than mercury. Select those that apply. Bromine is diatomic. Mercury is monoatomic. Diatomics are more ordered than monoatomics. Water has strong hydrogen bonding interactions. O Hydrogen-bonded substances are less ordered than substances without hydrogen-bonds.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY