
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
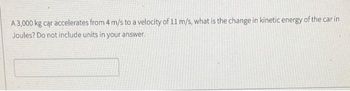
Transcribed Image Text:A 3,000 kg car accelerates from 4 m/s to a velocity of 11 m/s, what is the change in kinetic energy of the car in
Joules? Do not include units in your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the kinetic energy of a 0.15kg baseball moving at a speed of 45.0ms. Round your answer to 2 significant digits.arrow_forwardA 125 g piece of metal is heated to 288°C and dropped into 85 g of water at 12°C. The metal and water come to the same temperature of 24°C. What is the specific heat of the metal in joules over grams degrees Celsius?arrow_forwardWhat is the kinetic energy of a 23.1 g ball moving at 39.1 m/s? Report your answer with the correct number of significant figures. Do not include units in your answer.arrow_forward
- A) An iron nail with a mass of 12 gg absorbs 15 J of heat. If the nail was initially at 30 ∘C, what is its final temperature? Express your answer using two significant figures. B) When 47.1 J of heat is added to 13.8 g of a liquid, its temperature rises by 1.74 ∘C. What is the heat capacity of the liquid?arrow_forwardYou are asked to study how much table sugar can be mixed or dissolved in water at different temperatures. The amount of sugar that can dissolve in water goes up as the water’s temperature goes up. What is the independent variable? Dependent variable? What factor is held constant?arrow_forwardIf a glass of water and a lake have the same temperature, which has more thermal energy?arrow_forward
- A metal sample weighing 128.00 grams and at a temperature of 98.2 degrees Celsius was placed in 45.50 grams of water in a calorimeter at 22.8 degrees Celsius. At equilibrium the temperature of the water and metal was 35.6 degrees Celsius. What is the change in temperature of the metal (in degrees C)? (Report this as a positive value)arrow_forwardConstants I Periodic Table Part A How much heat must be absorbed by a 13.0 g sample of water to raise its temperature from 13.0 C to 40.0°C? (For water, C, = 4.18J/g C) Express your answer to three significant figures and include the appropriate units. Value Units Submit Previous Answers Request Answer X Incorrect; Try Again; 29 attempts remaining Provide Feedback acerarrow_forwardA heat pump is an electrical device that heats a building by pumping heat in from the cold outside. In other words, it's the same as a refrigerator, but its purpose is to warm the hot reservoir rather than to cool the cold reservoir (even though it does both). Let us define the following standard symbols, all taken to be positive by convention:Th = temperature inside buildingTe = temperature outsideQh heat pumped into building in 1 day Qe = heat taken from outdoors in 1 dayW = electrical energy used by heat pump in 1 day Explain why the "coefficient of performance" (COP) for a heat pump should be defined as Qh /W.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY