
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Answer please
Transcribed Image Text:Question 7 of 9
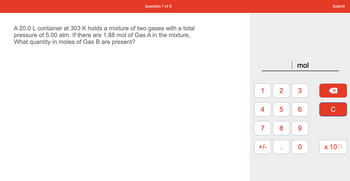
A 20.0 L container at 303 K holds a mixture of two gases with a total
pressure of 5.00 atm. If there are 1.88 mol of Gas A in the mixture,
What quantity in moles of Gas B are present?
1
4
7
+/-
2
LO
5
8
mol
3
CO
6
9
0
Submit
X
C
x 100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why is 7.28 R and 7.29 S? Because both of the 4 is on a line, and I thought that the configuration would only switch when the lowest priority was on the wedge.arrow_forward4arrow_forward4. Draw an energy level diagram for bromine esc F tab 7 caps lock shift 19 NO O 80 2 3 A #3 1 control 888 19 S N S4 6 E 1 option 25 (5 R D X 94 78 T F Y G 1 3: * 00 2 H B 8: - 0⁰ ✓ N $10 O O K M P L < -arrow_forward
- Answer allarrow_forwardidentify the groups of homotopic protons and from these, the group with the lowest and the group with the highest shift values. Give an estimate for these shift values.arrow_forwardWhich of the following IR frequencies would be expected for ethanol? Select all that apply. ~1710 cm¹ (broad) ~2870 cm 1 O~2250 cm 1 0-3075 cm ¹ ~3410 cm1 (broad)arrow_forward
- label major peaksarrow_forwardHow many 07 9 8 6 ¹HNMR 23) Reset Answer signals will observe for (2-bromopropyl)benzene?arrow_forwardConvert the following structural formulas into skeletal structures (or line formulas). Part 1 of 3 H H H-C-C-C H H Part 2 of 3 HICH x-U-x HHH H-C-C=C -C-H Click and drag to start drawing a structure. Click and drag to start drawing a || 9.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY