
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A 15.0 g sample of Cu(NO3)2.nH2O is heated. After the water has been driven off, 11.7 g of Cu(NO3)2 remains. What is the value of n in the empirical formula of this hydrate?
Expert Solution
arrow_forward
Step 1i
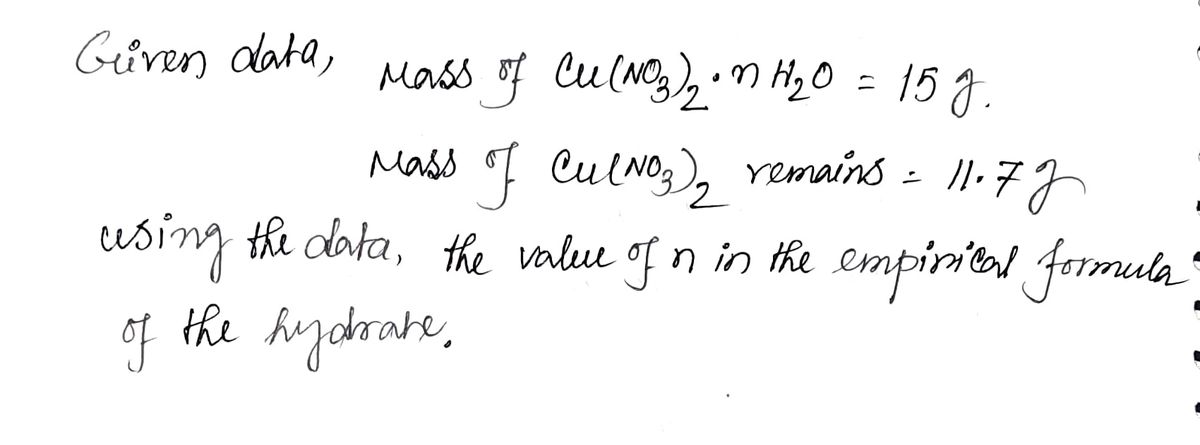
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Silver sulfide, Ag2S, also called argentite, is an economically important silver ore. If a silver mine extracts 38.0 kg of argentite per day, how many kilograms of silver will it produce per day? Molar Mass of Ag2S = 247.8 g/mol Molar Mass of Ag = 107.87 g/molarrow_forwardHow many moles of P 4 O 10 in 1.11 grams of P 4 O 10 ? [The Molar Mass of P 4 O 10 283.886 g/mol.]arrow_forwardMn3O4 is an unusual ionic compound in which some of the manganese ions have a charge of +2 and others have a charge of +3. A chemist reacts 18 grams of solid manganese with excess oxygen and allows the reaction below to proceed to completion: 3 Mn(s) + 2 O2(g) --> Mn3O4(s) The chemist collects 21 grams of Mn3O4 (228.8 g/mol). The yield of the reaction is _______%arrow_forward
- Determine the theoretical yield, in grams, of Fe2(CO3)3 produced from the complete reaction of 1.72 grams of Fe(NO3)3 with excess Na2CO3.arrow_forwardCryolite, Na3AlF6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. Balance the equation. 1.) Balance the equation - AlO3(s)+NaOH(l)+HF(g)-->Na3AlF6+H2O(g) 2.)If 16.4 kilograms of Al2O3(s), 60.4 kilograms of NaOH(l), and 60.4 kilograms of HF(g) react completely, how many kilograms of cryolite will be produced? 3.)Which reactants will be in excess, (Al2O3, NaOH, or HF) 4.)What is the total mass of the excess reactants left over after the reaction is complete in KG?arrow_forwardSmall quantities of oxygen can be prepared in the laboratory by heating potassium chlorate, KClO3(s). The equation for the reaction is 2KClO3⟶2KCl+3O2 Calculate how many grams of O2(g) can be produced from heating 12.2 g KClO3(s).arrow_forward
- Copper(II) sulfate forms several hydrates with the general formula CuSO4·xH2O, where x is an integer. If the hydrate is heated, the water can be driven off, leaving pure CuSO4 behind. Suppose a sample of a certain hydrate is heated until all the water is removed, and it's found that the mass of the sample decreases by 31.%. Which hydrate is it? That is, what is x?arrow_forwardCooper, Zach and Nate did this experiment dissolving 3.80 g of Cu(C2H3O2)2·H2O and adding 8.60 g NaC7H4SO3N·H2O (assume present in excess). What is the theoretical yield of the product, Cu(C7H4SO3N)2(H2O)4·2 H2O(s)? Molar Mass of Copper(II) acetate monohydrate = 199.65 g/mol Molar Mass of Sodium saccharinate monohydrate = 223.18 g/mol Molar Mass of product = 535.59 g/molarrow_forwardWhat mass of cobalt (III) sulfide is produced from the reaction of 0.750 g of sulfur with 3.50 g of cobalt? Co (s) + S (s) → Co₂S, (s) garrow_forward
- If 465 g of Pb(NO3)2 (FW 331.21 g/mol) is added to a solution containing 215 g of K3PO4 (FW 212.27 g/mol), what mass, in g, of Pb3(PO4)2 (FW 811.54 g/mol) precipitate will be formed? The balanced equation is 3 Pb(NO3)2(aq) + 2 K3PO4(aq) → Pb3(PO4)2(S) + 6 KNO3(aq)arrow_forward1) 2 Cu + S> Cużs When copper is heated with sulfur, the reaction above takes place. If 100. g of Cu is heated with 50.0 g of sulfur, which is the limiting reactant? What is the theoretical yield of Cu>S?arrow_forwardLook at the balanced chemical equation for the reaction between copper and nitric acid: Cu(s) + 4HNO3(aq) → Cu(NO3)2(aq) + 2H2O(l) + 2NO2 (g) How many grams of nitrogen dioxide would be produced if 2.47 g of copper were reacted with excess of nitric acid?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY