
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:A 100%
7:05 PM Wed Jan 29
Unanswered •1 attempt left • Due on Jan 30
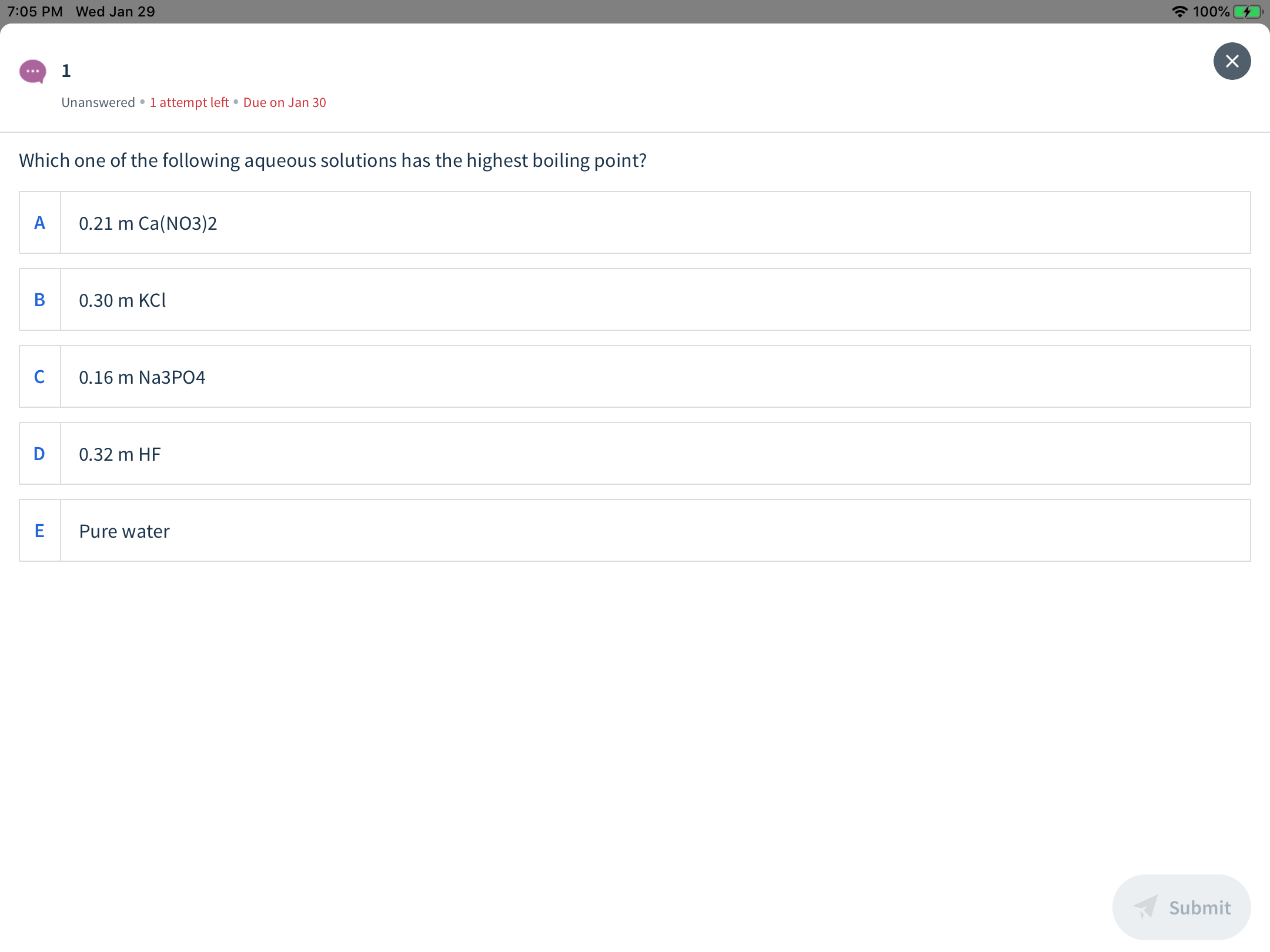
Which one of the following aqueous solutions has the highest boiling point?
0.21 m Ca(NO3)2
0.30 m KCI
0.16 m Na3PO4
D
0.32 m HF
Pure water
Submit
ш
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- arrow_forwardA 0.2585 gram amount of H₂C₂O4 is dissolved in approximately 100 mL of 0.5 M H₂SO4. A KMnO4 solution is added to a burette and added to the H₂C₂O₂ solution. A faint colour persists when 22.35 mL of KMnO solution has been added. slowly Calculate the molarity of the KMnO4 solution. A) 0.2294 M B) 0.02235 C) 0.05139 M 0.02927 Marrow_forwardWhat is the equilibrium concentration of N₂ in water that is in contact with air at 25 °C and air pressure 1 atm? The mole fraction of N2₂ in the air is 80 %, and Henry's law constant for N₂ is 6 x 10-4 mol L-1 atm-1. Select the nearest value. O 4.8x 10-3 mg L-1 4.8 x 10-4 mmol L-1 0.6 x 10-4 mol L-1 6 x 10-3 g L-1 6 x 10-4 mol L-1arrow_forward
- Solubility (mg/100 gH₂O) 10 8 6 4 2 0 10 20 Temperature NO 0₂ CO CHA 4 N₂ 2 30 40 (°C)arrow_forwardThe toxicity and solubility of an element (e.g., As, Cr, U) is never related to its oxidation state. True Falsearrow_forwardThese are six test tubes containing 0.1 M aqueous solutions of (in no particular order) (view attatchments) Match the following to each other: A= green solution B= blue solution C= red solution D= colorless solution E= colorless solution F = colorless solution and NaOH Ca(NO3)2 Cu(NO3)2 H2SO4 Co(NO3)2 Ni(NO3)2arrow_forward
- K2CO3(aq) + 2HC2H3O2(aq) yields 2KC2H3O2(aq) + H2O(l) + CO2(g) How many moles of H2O can be obtained from 25.0 ml of 0.150 M HC2H3O2?arrow_forwardDetermine the relative solubilities of the following compounds: Relative Solubility, Compound Ksp Mn(OH)2 4.6x10-14 MgCO3 4.0x10-5 Pb3(PO4)2 3.0x10-44 ✓ M lowest intermediate highest ✪ ↑arrow_forward18. What is the maximum mass of solid barium sulfate (MW = 233 g/mol) that can be dissolved in 1.00L of 0.250M Na2SO4 solution? (Ksp of BaSO4 = 1.5 x 10⁹) 01 xfarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY