
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
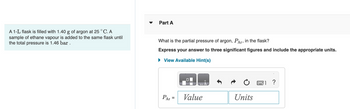
Transcribed Image Text:A 1-L flask is filled with 1.40 g of argon at 25 °C. A
sample of ethane vapour is added to the same flask until
the total pressure is 1.46 bar.
Part A
What is the partial pressure of argon, PAT, in the flask?
Express your answer to three significant figures and include the appropriate units.
► View Available Hint(s)
PAr
=
Value
Units
www.
?
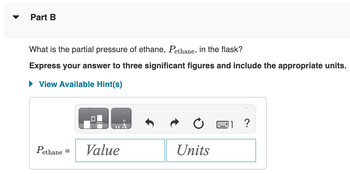
Transcribed Image Text:Part B
What is the partial pressure of ethane, Pethane, in the flask?
Express your answer to three significant figures and include the appropriate units.
► View Available Hint(s)
Pethane =
0
Value
Units
?
Expert Solution
arrow_forward
Step 1: Ideal gas equation
Answer:
Here:
Step by stepSolved in 4 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the temperature in K of 0.500 mole of neon in a 2.00L vessel at 4.32 bar? R = 0.08314 L· bar/mol·K.arrow_forwardConsider the following situation: You have a 50 L tank of compressed air at 15 atm and 100 ºC. You then pass the compressed air through a "titanium getter" which contains fragments of hot titanium metal. The purpose of the "getter" is to remove oxygen gas from the sample as Ti reacts with oxygen to form solid TiO2 (known as titania). Once the purified air is passed through the getter is it cooled back down to 100 ºC and fills another 50 L storage tank. If air typically contains about 20% oxygen, what could the pressure in the storage tank be? Question 2 options: A) 12 atm B) 15 atm C) 18 atm D) 24 atmarrow_forwardHot gaes Expanding A mixture hot gases expands, changing volume from 2.45 L to 6.82 L, against a constant pressure of 3.22 atm. The P-Vwork involved is Latm .arrow_forward
- a) From the temperature-pressure data graphed in Part A, visually determine the gas pressure at a temperature of 350 K, including units. (b)From the temperature-pressure data graphed in Part A, determine the equation of the line of best fit. (C) What are the units for the slope of the line of best fit determined in question 2? (D) From the equation of the line of best fit determined in Question 2, algebraically determine the gas pressure at a temperature of 350 K, including units and a unit analysis. Note: I only need su part D solve pleasearrow_forward11. (a) In a 20.0 L steel container, we have only 77.7 g of CO2(g), 66.6 g of N2(g), and O2(g). The temperature is 25.0 ◦C and the total pressure is 8.88 atm. What mass of O2(g) do we have, and what is its partial pressure? The molar masses of C, N, and O are 12.01, 14.01, and 16.00 g/mol. (b) The density of a sample of pure CH4(g) at a constant pressure of 1.00 atm is 0.666 g/L. What is the average speed, or root mean square speed, of the CH4(g) molecules in this sample? The molar masses of C and H are 12.01 and 1.01 g/mol.arrow_forwardUse your text or other reference to find the partial pressure of water at 25°C, 26°C, and 27°C and record those values.arrow_forward
- Please see question 28, just the top one. I figure in this system the pressure is changing and my answer is the original pressure divided by the final pressure is equal to X. Do I have this correct? pv= nRT N and T and R are constants thus P1V1 = P2V2.arrow_forward5 Calculate the mass of carbon monoxide gas (in mg) dissolved in 9.8L tank exposed to a pressure of 1.13 atm of air at 25˚C. Assume the mole fraction of carbon monoxide gas in air to be 0.112 given that the Kh for CO is 9.5 x 10-4 M/atm. Report answer to the correct significant figures with no units.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY