Question
1

Transcribed Image Text:please help on part C. please show the steps.
Thank you!
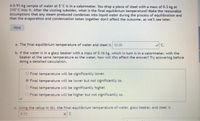
Transcribed Image Text:A 0.91-kg sample of water at 5°C is in a calorimeter. You drop a piece of steel with a mass of 0.3 kg at
210°C into it. After the sizzling subsides, what is the final equilibrium temperature? Make the reasonable
assumptions that any steam produced condenses into liquid water during the process of equilibration and
that the evaporation and condensation taken together don't affect the outcome, as we'll see later.
Hint
a. The final equilibrium temperature of water and steel is 12.05
C.
b. If the water is in a glass beaker with a mass of 0.16 kg, which in turn is in a calorimeter, with the
beaker at the same temperature as the water, how will this affect the answer? Try answering before
doing a detailed calculation.
O Final temperature will be significantly lower.
Final temperature will be lower but not significantly so.
O Final temperature will be significantly higher.
O Final temperature will be higher but not significantly so.
c. Using the setup in (b), the final equilibrium temperature of water, glass beaker, and steel is
973
x'C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- An airplane left an airport and flew 105 mi in the direction of North 50.4000 deg E, then turned and flew 493 mi in the direction of South 11.9000 deg E. How far (mi) and in what direction should it return to the the airport?arrow_forwardBour In figure (a) vector v shows the direction of a (+) positive charge moving with velocity v. The direction of the magnetic field B is also shown. What is the direction of the magnetic force (F) on the moving charge? In figure (b) vector v shows the direction of a (-) negative charge moving with velocity v. The direction of the magnetic field B is also shown. What is the direction of the magnetic force (F) on the moving charge? (a) (b) In figure (c) vector v shows the direction of a (+) positive charge moving with velocity v. The direction of the magnetic field B is also shown. What is the direction of the magnetic force (F) on the moving charge? x Ba B In figure (d) vector v shows the direction of a (-) negative charge moving with velocity v. The direction of the magnetic field B is also shown. What is the direction of the magnetic force (F) on the moving charge? In figure (e) vector v shows the direction of a (+) positive charge moving with velocity v. The direction of the…arrow_forwardIf the speed of the skateboarder at point A is v= 1.2 m/s, what is her speed at point B? Assume that friction is negligible. Express your answer using appropriate units. V Templates Symbols undo redo reset keyboard shortcuts help L Value Unitsarrow_forward
arrow_back_ios
arrow_forward_ios