
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
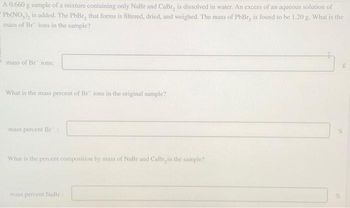
Transcribed Image Text:A 0.660 g sample of a mixture containing only NaBr and CaBr, is dissolved in water. An excess of an aqueous solution of
Pb(NO₂), is added. The PbBr, that forms is filtered, dried, and weighed. The mass of PbBr, is found to be 1.20 g. What is the
mass of Br ions in the sample?
mass of Br ions:
What is the mass percent of Br ions in the original sample?
mass percent Br
What is the percent composition by mass of NaBr and CaBr, in the sample?
mass percent NaBr
g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 34. Consider a mixture of the following ions in aqueous solution: NH,, Mg, Ni, Cl, S, and CO, Write the balanced net ionic equation(s) for any reactions that occur. (Hint: You have to consider nine combinations of cation + anion.)arrow_forward3. A solution contains silver nitrate, lead (II) nitrate, and calcium nitrate. What reagent could be added to this solution that would separate the calcium nitrate from the other three compounds? Write the formula of the resulting precipitates after the addition of this reagent.arrow_forwardDetermine the volume (in mL) of water that needs to be added to 50.0 mL of a 0.600 M LİBR to produce a 0.180 M solution. Assume the volumes are additive.arrow_forward
- What volume (in mL) of water must you add to 50.0 mL of a 0.600 M LiBr solution to produce a 0.185 M solution? Assume the volumes are additive.arrow_forwarda) What is the maximum amount of salt (in molecules) that can be produced when 35 mL of 0.102 M of strontium hydroxide reacts with 12 mL of 0.321 M nitrous acid? Does the reaction result in a basic, neutral or acidic solution?arrow_forward1. If 30.4 g of Nal (MM=149.89g/mol) are added to a 500.0 mL volumetric flask, and water is added to fill the flask, what is the concentration of Nal in the resulting solution? 2. What volume (in L) of 1.1 M FeCl3 would be required to obtain 0.59 moles of Cl- ions?arrow_forward
- if 38.0 g of KCl (MM=74.55 g/mol) are added to a 500.0 mL volumetric flask, and water is added to fill the flask, what is the concentration of KCi in the resulting solution?arrow_forward3. A student followed the procedure of this experiment to determine the percent NaOCl in a commercial bleaching solution that was found in the basement of an abandoned house. The student diluted 50.00 mL of the commercial bleaching solution to 250 mL in a volumetric flask, and titrated a 20-mL aliquot of the diluted bleaching solution. The titration required 35.46 mL of 0.1052M Na,5203 solution. A faded price label on the gallon bottle read S0.79. The density of the bleaching solution was 1.10 g mL-? h. Calculate the mass of one gallon of the commercial bleaching solution. i. Calculate the cost of 100 g of the commercial bleaching solution. j. Determine the cost of the amount of commercial bleaching solution required to supply 100 g of NaOCl.arrow_forwardAssume the given amount of RbF fully dissociates in water. Calculate the molarity of a solution if 145.20 g of RbF are dissolved into a solution of water that has a final volume of 2.00 L.arrow_forward
- 14.4 g of magnesium nitrate is dissolved in 1.20 L of water. 25 mL of this solution is taken and diluted to 175 mL with water. What is the final concentration of the nitrate ion?arrow_forwardb) The solubility rules provide a qualitative assessment of what combination of ions will form a precipitate when mixed in water. However, what general statement can be made regarding the solubility of all ionic compounds? Why? c) Explain why gases dissolve less in solution when the solution is heated?arrow_forwardA precipitation reaction involves the formation of a precipitate when aqueous solutions are mixed. Not all combinations of aqueous solutions produce precipitates, and it is important to be able to predict the ones that do. A) Complete and balance the molecular equation for the reaction between aqueous solutions of lithium fluoride and potassium chloride, and use the states of matter to show if a precipitate forms. B) Write the complete ionic equation for the reaction that takes place when aqueous solutions of lithium fluoride and potassium chloride are mixed. C) Write the net ionic equation for the precipitation reaction, if any, that may occur when aqueous solutions of lithium fluoride and potassium chloride are mixed. If there is no net ionic equation, simply write "none."arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY