
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
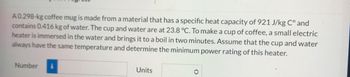
Transcribed Image Text:A 0.298-kg coffee mug is made from a material that has a specific heat capacity of 921 J/kg C° and
contains 0.416 kg of water. The cup and water are at 23.8 °C. To make a cup of coffee, a small electric
heater is immersed in the water and brings it to a boil in two minutes. Assume that the cup and water
always have the same temperature and determine the minimum power rating of this heater.
Number i
Units
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- The heating element of a 1200 Watt electric stove can reach temperatures of 815 °C when turned all the way up. If 86.0 % of the heat from the element is transferred into a pot of boiling water, how much water will boil off in one minute? 0.018 x kg Question Help: Message instructor Submit Questionarrow_forwardA power plant burns coal to produce pressurized steam at 554 K. The steam then condenses back into water at a temperature of 334 K If the plant operates at 50.0% of its maximum efficiency and its power output is 1.23 * 10 ^ 8 * v means of a cooling tower ? at what rate must heat be removed by means of cooling tower?arrow_forwardA 500-W, 230-volt electric kettle contains 1.5 kilograms of water. Determine the thermal efficiency of the kettle if the water will boil after 15 mins.arrow_forward
- It uses an underground lake as the cold reservoir of a Carnot heat pump that maintains the temperature of a science lab at 318.5 K. To deposit 11346 J of heat in the lab, the heat pump requires 1108 J of work. Determine the temperature of water in the underground lake, in Kelvin.arrow_forwardA small metal cube with a thermal mass mc and an initial temperature 0 is dropped into a container of water that is actively maintained at a constant temperature 0w. The cube quickly comes to rest on the bottom surface of the container. The bottom surface is maintained at a constant temperature 0 (note that this is different from 0w). The thermal resistance between the cube and the water is Rcw while the thermal resistance between the bottom surface of the container and the cube is RCB. The temperature of the cube is denoted by 0c. a) Draw the system schematic indicating the assumed directions of the heat transfer rates. Label all the nodes and system parameters. b) Derive the governing equation for the temperature of the cube, 0c. c) Where does to appear in the system schematic and how does it affect the governing equations? d) Calculate the steady-state temperature of the cube, css, assuming the following system parameters: 0o = 21°C, 0B = 6°C, 0w = 0°C, Rcw = 2°C/W, and RCB = 4°C/Warrow_forwardA heat pump moves heat from the chilly 8°C air outside a house to the toastier 21°C air inside. The actual coefficient of performance of the system is 3.2, a typical value. How much energy would it take to add 11 kJ each second if the heat pump ran at the maximum theoretical efficiency?arrow_forward
- In the comparison of heat and electrical energy flowing along a well insulated conductor which of these is/are true? 1) The Pd across the ends of the conductor is analogous to the temperature difference 44. between the ends of the conductor. 2) The electric current is analogous to the rate of flow of heat 3) The electrical resistance is analogous to the of thermal conductivityarrow_forwardQuestion 10. A detective has been called to the scene of a crime where a dead body has just been discovered. The detective arrives at 9:00 AM and begins to investigate. Immediately, the temperature of the body is taken and is found to be 90.3°F. The detective checks the programmable thermostat and finds that the room has been kept at a constant 68.0°F for the past 3 days. After the evidence from the crime scene is collected, the temperature of the body is taken once more and found to be 89.0°F. This last temperature reading was taken exactly one hour after the first one. The following day, the detective is asked "At what time did our victim die?" Assuming that the victim's body temperature was normal (98.6°F) prior to death, what should the detective answer?arrow_forwardThree 105.0-g ice cubes initially at 0oC are added to 0.900kg of watter initially at 21.5oC in an insulated container. What is the mass of unmelted ice, if any, when the system is at equilibrium?arrow_forward
- A 440 kW of reftigerator working in a room at 35 °C and rejects 450 kj/s. Extimate the temperature of the cooling space.arrow_forwardA 1200 W electric heater has 750 L of water at 100°C in it. How much time will it need to vaporize half of its content? O 10 minutes O 12 minutes O 16 minutes O 14 minutesarrow_forwardA certain coal-fired power plant has a rated power capacity of P = 850 MW. The output of this plant is W = 0.21QH, where QH is the energy input as heat from the hot reservoir. a) Which would increase the efficiency more, doubling QH or reducing QC by half? b) Calculate the maximum thermal efficiency of the power plant. c) Calculate the absolute value of the exhausted heat (QC) each second in MJ for the power plant. d) If the power plant operates for a full day at its rated capacity, how much energy QH in MJ is needed? e) If, on average, one ton of coal contains Q = 25 GJ of energy, how many tons nH of coal would the plant need to operate for a day at its rated capacity? f) How many tons nC of this coal is exhausted as wasted heat to QC in a single day?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON