
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
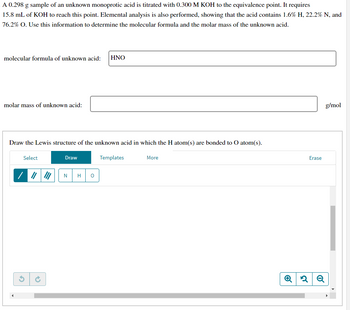
Transcribed Image Text:A 0.298 g sample of an unknown monoprotic acid is titrated with 0.300 M KOH to the equivalence point. It requires
15.8 mL of KOH to reach this point. Elemental analysis is also performed, showing that the acid contains 1.6% H, 22.2% N, and
76.2% O. Use this information to determine the molecular formula and the molar mass of the unknown acid.
molecular formula of unknown acid: HNO
molar mass of unknown acid:
Draw the Lewis structure of the unknown acid in which the H atom(s) are bonded to O atom(s).
Select
3
Draw
N H O
Templates
More
Erase
Q2 Q
g/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Step 1: Define equivalence point of titration between acid and bases
VIEW Step 2: Determine molar mass of unknown acid
VIEW Step 3: Calculate empirical formula of unknown acid
VIEW Step 4: Determine molecular formula of unknown acid
VIEW Step 5: Draw Lewis structure of unknown monoprotic acid
VIEW Solution
VIEW Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A titration is carried out for 25.00 mL of 0.100 M HCI (strong acid) with 0.100 M of a strong base NaOH (the titration curve is shown in Figure 14.18) Calculate the pH after the addition of 30.00 mL base.arrow_forwardA student is titrating a 100 mL of 0.50 M solution of hydrofluoric acid (HF) with 1.0 M strong base (NaOH). a) at the equivalence point, what ions/compounds will be present in the solution? A complete answer will also be specific as to what ions/compounds are not present in solution. b) calculate the pH at the equivalence point.arrow_forwardYou measure 0.210 gram of unknown diprotic acid into a beaker. You add distilled water and a chemical indicator. You slowly add titrant 0.100M NaOH acid solution. Your first equivalence point is reached after 15mL NaOH has been added. The second equivalence point is reached after 30.0mL. WHat is the molar mass of the unkown diprotic acid?arrow_forward
- A solution of a weak acid is titrated with a standard solution of a strong base. The progress of the titration is followed with a pH meter. Which of the following observations best describes what would occur? Initially the pH of the solution increases slowly, and then it increases much more rapidly, before increasing slowly again At the equivalence point, the pH is 7 The pH of the solution gradually decreases throughout the experiment After the equivalence point, the pH becomes constant because this is the bufferarrow_forwardPlease only answer the question below. The questions shown are already solved, you don’t need to add anything, they are there for context. 3) Calculate the [Ba2+] left in solution when Ca2+ starts to precipitate. Then calculate the % of the original Ba2+ left in solution when Ca2+ starts to precipitate. A) 1.1 • 10^-2 % B) 2.8 • 10^-7 % C) 9.3 • 10^-3 % D) 6.7 • 10^-3 % E) 5.6 • 10^-3 %arrow_forwardtheoretically, the equivalence point and end point of a titration should be the same. Describe four factors that could be responsible for the difference between these two in an actual titrationarrow_forward
- Question 6 (1 point) 14 12 10 8 PH 00 6 4 2 0 0 20 40 60 volume added (mL) Consider the graph. What type of titration does it represent? A strong base titrated with a strong acid. A weak acid titrated with a strong base. A weak base titrated with a strong acid. A strong acid titrated with a strong base.arrow_forward(Answer highlighted questions please) A 50.050.0 mL solution of 0.1390.139 M KOHKOH is titrated with 0.2780.278 M HClHCl. Calculate the pH of the solution after the addition of each of the given amounts of HClHCl. 20.0 mLpH=20.0 mLpH= 24.0 mLpH=24.0 mLpH= 25.0 mLpH=25.0 mLpH=arrow_forwardSelect ALL of the following that can be determined from the titration of a strong, monoprotic acid with a standardized solution of sodium hydroxide? moles of acid in the titration moles of hydroxide ions used in the titration mass of acid titrated | volume of sodium hydroxide used in the titration Select ALL of the following quantities that must be used in a titration calculation to find the identity of an unknown strong acid: concentration of the standardized base solution stoichiometric ratio of acid to base volume of the acid solution used in the titration volume of the base used in the titration at the equivalence point Need Help?arrow_forward
- A 0.338-g sample of an unknown triprotic acid is titrated to the third equivalence point using 33.4 mL of 0.163M NaOH. Calculate the molar mass of the acid. Question 1 options: A) 5.44 g/mol B) 1.86× 102 g/mol C) 20.7 g/mol D) 69.3 g/mol E) 62.1 g/molarrow_forward3. A 0.3535 g sample of KHP requires 19.27 mL of NaOH to reach the end point. What is the concentration of NaOH? 4. What properties should the indicator have for titration of KHP with NaOH?arrow_forwardRegarding titrations, which of the following is TRUE? A plot of pH vs. the [base]/[acid] ratio is known as a titration curve. The equivalence point is when the moles of base equals the moles of acid during any acid–base titration. Indicators do not respond to changes in pH. The pH is always 7 once the equivalence volume has been delivered. None of the answers are true.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY