
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Rank both sets of compounds in order of increasing oxidation level:
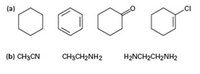
Transcribed Image Text:(a)
.CI
(b) CH3CN
CH3CH2NH2
H2NCH2CH2NH2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following reaction may be classified as an oxidation-reduction reaction. Which of the species which is reduced and explain why. C2H6(g) + O2(g) ⟶⟶CO2(g) + H2O(g) A) carbon, it gains electrons B) carbon, it loses electrons C) oxygen, it loses electrons D) oxygen, it gains electronsarrow_forwardWhich of the following reactions is neither an oxidation nor a reduction reaction. A. NH3 > N204 B. S203? > SĄ0G² C. SO3 → HSO4 D. N2O4→ NO2arrow_forwardWe will find the vitamin C concentration of fruit juice by oxidizing ascorbic acid. Write the oxidation half-reaction and reduction half-reaction.arrow_forward
- 7A-5. For each of these single displacement reactions:(1) Write a balanced molecular equation, including all physical states. Use the solubility rulesprovided on the Course Resources page.(2) Write a balanced total ionic equation, including all physical states and charges forindividual ions.(3) Write a balanced net ionic equation, including all physical states and charges forindividual ions.(4) State what is oxidized and briefly explain how you can tell.(5) State what is reduced and briefly explain how you can tell.a. barium and manganese(II) acetatearrow_forwardThe oxidation of oxgen in the first photo the oxidation of teh ble and red in the seceong photo. with explanation plesearrow_forwardWhat is the oxidation number of Oxygen in oxygen difluoride. Be sure to write a plus (+) or minus (-) before the number with no space between.arrow_forward
- O3- is an oxidizing agent that oxidizes : KI to I2 I2 to I- S2O3- to Iarrow_forwardMatch the term with its definition. The loss of one or more electrons. A laboratory procedure in which a substance in a solution of known concentration is reacted with another substance in a solution of unknown concentration (or quantity) to determine unknown concentration (or quantity). 1. Oxidation The gaining of one or more 2. Reduction electrons. Reactions in which 3. REDOX reaction clectrons are transferred from one reactant to 4. Percent by mass another and the oxidaton states of certain atoms are 5. Indicator change. 6. Titration An dye whose color depends on the pH or other chemical state of the solution it is dissolved in; it changes color at the end of a titration expcriment. A unit for expression solution (mixture) concentration in parts by mass with a multiplication factor of 100%. 00arrow_forward2 OH- + AO3-+2BO2 -> A- +2 BO4-+H2O Fine the oxidized, reduced, species and agents. A is a halogen and B is a Metalarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY