
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
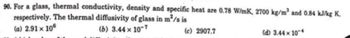
Transcribed Image Text:90. For a glass, thermal conductivity, density and specific heat are 0.78 W/mK, 2700 kg/m³ and 0.84 kJ/kg K,
respectively. The thermal diffusivity of glass in m²/s is
(a) 2.91 x 106
(b) 3.44 x 10-7
(c) 2907.7
(d) 3.44 x 104
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Analyze the following diagram. What is the heat rate to the left side of the heater? What is the heat rate to the right side of the heater? Tin = 16°C W h = 40- T = -20°C u = 188 m2K 60mm Air 60mm 10mm 6mm 10mm 14mm 6mm 2mm 2mm Blue: Epoxy Grey: Aluminum Green: Fiberglass Yellow: Silicone Orange: Heater Blanket, 2000w Note: Treat the outer left wall as flat plates in external convection. Note: Ignore the thickness of the heater blanket.arrow_forward3 An electrical current of 700 A flows through a stainless steel cable having a diameter of 5 mm and an electrical resistance of 6 x 104 /m (i.e., per meter of cable length). The cable is in an environment having a tem- perature of 30°C, and the total coefficient associated with convection and radiation between the cable and the environment is approximately 25 W/m².K. (a) If the cable is bare, what is its surface temperature? (b) If a very thin coating of electrical insulation is applied to the cable, with a contact resistance of 0.02 m² K/W, what are the insulation and cable surface temperatures? (c) There is some concern about the ability of the insula- tion to withstand elevated temperatures. What thick- ness of this insulation (k = 0.5 W/m .K) will yield the lowest value of the maximum insulation temper- ature? What is the value of the maximum tempera- ture when this thickness is used? Note: The 25 W/m²K includes not only convection but also radiation.arrow_forwardEstimate the thermal conductivity of benzene at 30C.arrow_forward
- Answer: 24.187 °Carrow_forwardCalculate the energy associated with a radiation having wavelength 3000 Å. Give the answer in kcal mol-¹ and also in kJ mol-¹.arrow_forwardÀ wall of area 30 m² having a density of 1500 kg/m', thermal conductivity 30 W/m.K, and specific heat capacity 4 kJ/kg.K. The temperature distribution across a wall 0.5 m thick at a certain instant of time is given as T(x) = 30-5 x-7x The wall is generating a uniform heat (q.) of 1000 W. (1) Find the rate of heat transfer entering and leaving the wall (in W). (2) Find rate of energy stored in Watt. (3) Find (dFT/dx²) (4) Derive the change in temperature with respect to time equation (time rate of temperature change)- remember to substitute the value of (d T/dx²) from (part 3) and values of all other properties into final equation. %3Darrow_forward
- 2-2 A certain material 2.5 cm thick, with a cross-sectional area of 0.1 m², has one side maintained at 35°C and the other at 95°C. The temperature at the center plane of the material is 62°C, and the heat flow through the material is 1 kW. Obtain an expression for the thermal conductivity of the material as a function of temperature.arrow_forwardA piece of beef steak 7 cm thick will be frozen in the freezer room -30 ° C. This product has a moisture content of 73%, a density of 970 kg / m³, and a thermal conductivity (frozen) of 1.1 W / (m K). Estimate the freezing time. using the Plank equation. This product has an initial freezing temperature of -1.75 ° C, and the movement of air in the freezing room gives a convective heat transfer coefficient of 15 W / (m² K). t f = ... hour.arrow_forwardProblem 8. Energy is generated uniformly in a 20cm thick wall the steady state temperature distribution of the wall is indicated on the table below; z (cm) T (C) 8 10 12 14 16 18 20 0 2 4 6 150 177 205 230 250 272 292 310 330 345 360 If thermal conductivity of the wall is 15 W/m.K and the indicated temperatures are in °C, determine the average rate of heat generation in unit volume.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The