
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Briefly explain based on the IR spectra how well the reaction worked.
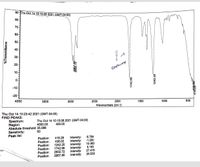
Transcribed Image Text:90-Thu Oct 14 10:15:08 2021 (GMT-04:00)
80-
70-
60-
50-
40-
30
20
10-
0-
-10-
-20-
4000
3500
3000
2500
2000
1500
1000
500
Wavenumbers (cm-1)
Thu Oct 14 10:23:42 2021 (GMT-04:00)
FIND PEAKS:
Spectrum:
Region:
Absolute threshold: 35.088
Sensitivity:
Peak list:
Thu Oct 14 10:15:08 2021 (GMT-04:00)
4000.00
400.00
50
Position:
Position:
Position:
Position:
Position:
Position:
416.28
436.02
1242.20
1742.66
2932.72
2957.85
Intensity:
Intensity:
Intensity:
Intensity:
Intensity:
Intensity:
-6.784
-1.200
10.063
6.163
27.416
28.539
mp
2
%Transmittance
1742.66
1242.20
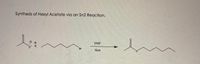
Transcribed Image Text:Synthesis of Hexyl Acetate via an Sn2 Reaction.
DMF
K
Br
Heat
Expert Solution
arrow_forward
Step 1
An organic reaction can be well monitored by the help of IR spectroscopy provided there is a significant change in the functional group. Depending on the bond stretching we can easily conclude whether a reaction is complete or not.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardWhich of the following compounds has a UV spectroscopic signal at the lowest absorption energy? II IV A. I B. II C. II D. IVarrow_forwardUse the Text Submission box to answer the following question. Consider the reaction shown below. For a particular concentration of sodium methoxide, the absorbance at 244 nm is measured using UV-vis spectroscopy. Over an initial period, the absorbance at 244 nm increases from 0.50 to 0.60. When the concentration of sodium methoxide is doubled, the absorbance at 244 nm measured after the same initial period is 0.70. What can you conclude about the mechanism of the reaction? NaOCH 3 Amax = 244 nmarrow_forward
- What happens to emission intensity when KI is added to a fluorescein solution and why?arrow_forward7. Explain how you could tell them apart, both by mass spectrometry and by infrared spectroscopy. IR values and MS fragmentations H 120 7arrow_forwardWith reference to the provided IR spectra, answer the following questions.arrow_forward
- 2. For the reaction performed in this experiment, the IR spectra for the reactants and product are shown below. For the IR spectra of acetone and the product, identify the important IR signals for each spectrum. Specifically: - for the IR spectrum of acetone, identify the signal which indicates the presence of the C=O bond and the signal which represents the CH bond (indicate the hybridization of the carbon). -for the IR spectrum of the product indicate which signal represents the CH bond (hybridization?) and which signal represents the O-H bond. You may write the answers on the spectra if you wish. Reactant: 1-bromobutane % Trasm ittaice 100 % Transmittance 20 GO 40 20 0 4000 Reactant: acetone 100 DO GO 40 20 3600 4000 3500 3000 IAN 3000 2500 M wavenumber (cm) 2500 2000 n 2000 Wavenumber (cm³¹) 1500 VI 1500 1000 1000arrow_forwardChapter 18 Worksheet 18-1 Properties of Light 1. The stretching frequency of a carbonyl (C=0) bond in a typical ketone is 5.15 x 1013 Hz. The vibrational energy of the molecule increases when light of this frequency is absorbed. a) What is the wavelength of this light in nanometers? b) What is the wavelength of this light in micrometers? c) Express the frequency of this light in wavenumbers. d) What is the energy (in joules) of a single photon of light at this frequency? e) What is the energy (in kilojoules) of one mole of photons at this frequency? f) Into what region of the electromagnetic spectrum does this radiation fall?arrow_forwardSample #1 has an absorbance of 0.300. Sample #2 is the same substance and has an absorbance of 0.600 using the same equipment. What is the relative concentration of the second sample? Select one: a. The concentration of sample #2 is the square root of that of #1. b. The concentration of sample #2 is that of sample #1 squared. c. The concentration of sample #2 is half that of sample #1. d. The concentration of sample #2 is twice that of sample #1.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY