
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
9. Uranium-235 undergoes nuclear fission as shown in the diagram below. a) Select which element does X represent? b) On your notes, write the complete formula for the nuclear reaction to include the chemical symbols with atomic numbers, mass numbers, and the proper coefficients for the neutrons.
Kr-98
Kr-96
Kr-95
Kr-97
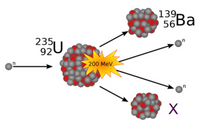
Transcribed Image Text:139
56BA
235
92
200 MeV
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Short Response. Directions: Write a balanced nuclear equation for the alpha and beta decay of each of the following II. A. Alpha Decay 1. 3No 2. 25Cf 251 102 98 B. Beta Decay 1. Strontium-92 2. Potassium-42arrow_forward5. Krypton-85 is an inert radioactive noble gas with a half-life of approximately 10.76 years. a. Write an exponential function to model this situation and define each variable. ( b. Determine what fraction of a sample of Krypton-85 will remain after 86.08 years. State the answer as an exact fraction value. ( C. Determine how long will it take for a sample of Krypton-85 to decay to 1/64 of its original mass. Round your answer to the nearest hundredth of a year.arrow_forwardQuestion 11 The most used 'nuclear fuel" for nuclear fission is Uranium-235. a) Explain what happens to a Uranium-235 nucleus when it undergoes nuclear fission. [Suggested word count 70] b) Write an equation and explain chain reaction and critical mass. (About 80 words) [4.2] (ROMEIO Uranium-235 TERSEB HAUTRUMAS BRGHINI PIGMERIN Januar HERRA PRAMON RACHUNKANI Smart Hom CASTROENTEROLEH Buntate-defen MEDIKAMETESTRANA MARDIRHAMMASRUTUBIG VENENARISTRERADE INCENGINES MAMAYE MIESTORCENT HORORE 6790EFATEST HELADINA MAGNASTOST Batman Thamasikan AGAINSIZE musmetentiel nucleus BATOTOHATROIS Th VERMENSENTRA LITERATU ZIR FUNGO24 RUSTRATE BINACZOGGE AMARANGHIENG PUIGHUSHANBE BABILMESA MATEMOHONRAKE DOCENTEKEN PAMISETAMOR QUEMESTASKERAT MIGZAMABESH unteffantennes INSTURISTERIOS DREOMINIAL Internat PARAN P FESTOONBASSINGENEZAMANTESCHICOTCH BROTHERS BRUGE LISTRUALESATARESSAASTE RUCHUONEISSENFUSION USTAREFASORASTENEarrow_forward
- 5. Krypton-85 is an inert radioactive noble gas with a half-life of approximately 10.76 years. a. Write an exponential function to model this situation and define each variable. ( b. Determine what fraction of a sample of Krypton-85 will remain after 86.08 years. State the answer as an exact fraction value. ( C. Determine how long will it take for a sample of Krypton-85 to decay to 1/64 of its original mass. Round your answer to the nearest hundredth of a year.arrow_forward3D. Write the equation for the alpha decay of Thorium-232. b) Write the element that is formed in the alpha decay of Thorium-232. Your answerarrow_forward4. Technitium-99m* is a gamma-emitter. Write the balanced nuclear equation for y-decay of technitium- 99m*. Note that m* means that this isotope is in a high-energy state and releases energy in the form of gamma radiation.arrow_forward
- Question 3 pleasearrow_forwardhttps://www.walter-fendt.de/html5/phen/decaychains_en.htm 1) Thorium series. Click on “next decay” to follow the decay chain of Thorium.a. Write down the full decay chain.b. How many alpha decays and how many beta decays are involved?2) Neptunium series. Click on “next decay” to follow the decay chain of Neptunium. What is the enddaughter nucleus for this decay chain?arrow_forwardWhich of the following describes safeguards used in nuclear power plants to protect the environment? I. Radioactive sections of the plant are cleaned every week. II. Cooling the reactor prevents an explosion and leak of radiation. III. A mixture of different forms of uranium prevents explosions. O l only Ol and II O Il and III O , II, and IIIarrow_forward
- 1. Determine the element created, and its atomic number and mass number when Lead-204 undergoes alpha decay, producing a stable isotope. Write the nuclear reaction equation for this alpha decay. lead 204 => 204 82 Pbarrow_forwardWhich of the following statements about nuclear fission is true? a. No new elements can be produced in a fission reaction. b. Energy released in fission reactions is generally less than that from fusion reactions. c. In a fission reaction, two light nuclei are combined into a heavier one. d. Fission reactions can be explained on the basis of the conservation of mass-energy.arrow_forward10. In the Sun, a series of nuclear reactions have the net effect of making one helium atom form four hydrogen atoms. Which process does this describe? (A) chain reaction (B) fission (C) fusion (D) nuclear reactorarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON