
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
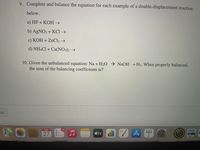
Transcribed Image Text:9. Complete and balance the equation for each example of a double-displacement reaction
below.
a) HF + KOH →
b) AGNO3 + KCI →
c) KOH + ZNC12 →
d) NHẠCI + Ca(NO3)2 →
10. Given the unbalanced equation: Na + H2O → NaOH +H2. When properly balanced,
the sum of the balancing coefficients is?
us
SEP
28
27
étv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Use the activity series to complete the following single replacement reaction. Choose the answer that best completes the reaction. Mg(C2H3O2)2 (aq) + Cu (I) (s) →arrow_forwardWhat are the products from the following single-replacement reaction? Mg(s) + HNO3(aq) →arrow_forward2. Write the molecular equation, the total ionic equation, and net ionic equation for the reaction of potassium hydrogen phthalate (KHP) with sodium hydroxide.arrow_forward
- Complete and balance each gas evolution reaction. 1. HBr(aq)+NaHCO3(aq)→ 2. NH4I(aq)+KOH(aq)→ 3. HClO4(aq)+K2SO3(aq)→ 4. HI(aq)+K2S(aq)→arrow_forwardComplete each of the following chemical equations.arrow_forwardFor the following reaction, do the following: 1. Write the correct formulas for the products, assuming a metathesis/exchange/double replacement/double displacement reaction is occurring 2. Using a solubility table, predict whether the reaction would produce a precipitate and fill in the phase subscripts for each product 3. Balance the equation, giving the correct balanced molecular equation 4. Write the total ionic equation 5. Write the net ionic equation FeCl3 (aq) + K2S (aq) →arrow_forward
- Determine which two of the following reactions are redox reactions. If the reaction is a redox reaction, indicate which element is being oxidized. Briefly explain your answers. Mg + Cl2 ⟶ MgCl2 MgO + 2HCl ⟶ MgCl2 + H2O 2CuCl2 + 4KBr ⟶ 2CuBr + 4KCl + Br2arrow_forwardClassify the following chemical reaction equation: 2 H N O 3 + C a ( O H ) 2 → C a ( N O 3 ) 2 + 2 H 2 Oarrow_forwardFor the complete redox reactions given here, write the half-reactions and identify the oxidizing and reducing agents. Make sure your answer has the simplest coefficients. 4Fe + 3O2 → 2Fe2O3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY