
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
9Q
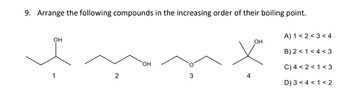
Transcribed Image Text:9. Arrange the following compounds in the increasing order of their boiling point.
fun
OH
2
OH
3
OH
A) 1<2<3<4
B) 2 <1 <4 <3
C) 4 <2<1 <3
D) 3 <4 < 1<2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1P Arrange the following in order of increasing boiling point: RbF, CO2, CH3OH, CH3Br. O CO2 < CH3OH < CH3Br < RbF O CH3B < CO2 < RbF < CH3OH O RbF < CO2 < CH3OH < CH3BR O CH;Br < CO2< CH3OH < RbF O CO2 < CH3Br < CH3OH < RbFarrow_forward7. Which of the following compounds has the highest boiling point? (1) Н-0 (2) H2S (3)H2Se (4)H,Tearrow_forwardRearrange the in order of lowest - highest boiling point Ch3COOH, (CH3)2C=O, CH3CH2CH2CH3arrow_forward
- Organic chemistryarrow_forwardPlace the following molecules in order from lowest boiling point to highest boiling point, according to their intermolecular forces. Briefly explain your choices, using specific IMF vocabulary.arrow_forward4. For this question use the following multiple choice answers: (A) (i) (ii) (iii) (iii) (i) (ii) < (a) Rank in increasing order of increasing boiling point OH A BC DE F OH (i) (ii) (iii) (b) Rank the following compounds in order of increasing boiling point: НО OH OH ABC DE F Но (i) (ii) (iii) (c) Rank in increasing order of number of unpaired electrons (i) Ne3+ (ii) Fe3+ (iii) Ga³+ A BC DE F (d) Rank the following compounds in order of increasing lattice energy: (i) Al(NO:)3 (ii) Li2S (iii) LiBr A BC DE F (e) Rank the INTERIOR F-X-F angle in increasing order (X is the central atom): (i) PF6 (ii) SİF4 (ii) KRF2 A BC DE F V V v v y varrow_forward
- TAGVICV COnStan The figure shows a line-angle six-carbon structure with an OH group attached to the sixth carbon atom. Part A Rank the following compounds in order of increasing boiling point (lowest to highest). Rank the compounds from the lowest boiling point to the highest boiling point. OH P Pearson 2019 Pearson Education Inc. All rights reserved. | Terms of Use Privacy Policy I Permissions Co Copyright Deuvery TT TNL C CTID NEL Only Only 2 left in stock - order soon. 30,639 OCT W 28arrow_forward3. The following 4 compounds; CH₁, CH₂Cl, and CH,OH, are very similar in structure, but have very different boiling points. Explain why. Iarrow_forwardIdentify the following compound(s) havingthe lowest boiling point? ОТ O II ||| IV III and IV н Ну CH ннн с н H-C-C-C-C-C-H Н Н Н Н ННННН Н-С-С-С-С-С-н НН Н нанн Н-С-С-С-Н нон HTH нннннн н-с-с-с-с-с-с-н H IVarrow_forward
- 3. List the types of intermolecular forces present in the following compounds. (a) HF (b) Вrz (c) H2S н (d) н—с-о-н Н нн нн (d) н-с—с—о—С—с-н нн ннarrow_forward3. Predict the correct order of the following compounds from lowest to highest boiling point at 1.00 atm pressure. Сompound 1 Сompound 2 Compound 3 он O A) (1) < (2) < (3) O B) (2) < (3) < (1) O () < (3) < (2) O D) (3) < (2) < (1) CO E) (3) < (1) < (2)arrow_forwardPleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning