
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
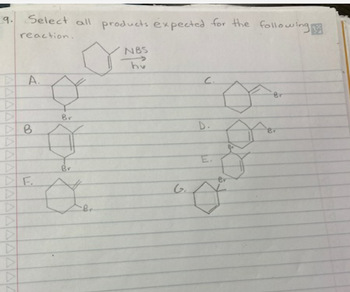
Transcribed Image Text:9.
A
Select all products expected for the following
reaction.
A.
B
F.
Br
Br
o
NBS
hv
C
G.
D.
M
Ø
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- This reaction (CO + .OH à CO2 + H) represents: A. Reducing the carbon monoxide in the air. B. A and B are correct. C. Reducing the carbon monoxide in the water. D. Producing hydrogene.arrow_forwardAnswer question in pictures pleasearrow_forwardWhich of the following is/are possible products of the reaction shown? HCI Product(s) CI А. .CI CI D. .CI Both B and D could possibly form. Only B could form. Both B and C could form. Both A and B could form. B. C.arrow_forward
- Nonearrow_forward8. Predict the product for the following reaction. NH₂ 1. excess CH₂l whom | A. I B. II 2. Ag₂O, H₂O heat || C. III D. IV N(CH3)2 E. V IV OH NHCH3arrow_forwardHeroin and morphine (shown below) are odorless solids. Drug-sniffing dogs can sometimes locate heroin that has been exposed to the elements because of a strong odor that is present in small quantities. What compound causes the odor and suggest what reaction is responsible for the compound's formation. H3C. но reaction? odor compound? 'N. CHз CHз H3C но herion morphinearrow_forward
- Three moles of sodium carbonate are mixed with two moles of lead nitrate in aqueous solution, leading to formation of a solid precipitate. How many moles of spectator ions remain in solution, assuming 100% yield of the precipitate? A. 12 B. 10 C. 5 D. 6 E. 11arrow_forward79arrow_forwardAccording to your knowledge, the major product of the following reaction is: SNa "Br 1) S 2) S 3) S 4) S a. 3 b. 4 C. 1 d. 2arrow_forward
- 7. How many moles of ions are produced if 1 mole of LizSO4 is dissolved in water? C. 3 a. 1 b. 2 d. 4 e. 5arrow_forwardThanks.arrow_forward3. PROVIDE FINAL PRODUCTS CI 1. Br2, hv 2. CzHz, NaNH, 3. BH3-THF then H₂O2, NaOH 4. Vinyllithium, Et₂0 5. H3PO4, A fo 1. 2. HBr LiCu 3. Mgº, Ether 4. CO2, then H30+ 2 ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY