
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
#9 please
Transcribed Image Text:e Chapter 19- Thermodynamics - x
ckboardcdn.com/blackboard.learn.xythos.prod/5970c46bc1741/9717426?X-Blackboard-Expiration=1650261600000.. 2 *
le...
O*NET OnLine
Ec Home | exploreheal..
Scholarships - SAL. Patient Portal - heal..
20 Highest Paid No...
2/ 2
100%
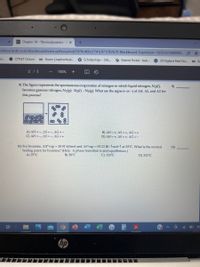
9) The figure represents the spontaneous evaporation of nitrogen in which liquid nitrogen, N2(),
becomes gaseous nitrogen, N28): N2(1) - N2(g). What are the signs (+ or -) of AH, AS, and AG for
this process?
9
A) AH =-, AS =-, AG=-
C) AH =-, AS = -, AG=+
B) AH = +, AS = +, AG = +
D) AH = +, AS =+, AG =-
10) For bromine, AH°vap = 30.91 kJ/mol and AS°vap = 93.23 JK-1mol-1 at 25°C. What is the normal
boiling point for bromine? (Hint: A phase transition is and equilibrium.)
A) 25°C
10)
B) 58°C
C) 124°C
D) 332°C
O 4)
hp
日
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In your own words, describe why metals are such good conductors of electricity. Be sure to include the definition of metallic bond in your answer. Answer in 2 to 3 complete sentences. I !!! H Normal A Enter your answer here BIUS √x 20 Txarrow_forwardFor each organic reaction, say whether at equilibrium there will be more reactants than products (so equilibrium lies to the left), or more products than reactants (so equilibrium lies to the right). You'll probably find some useful information in the ALEKS Data resource. OH OH + H30 Oleft + H3O + + + + H₂O left left OH right НО. right right OH₂ + + H₂O + OH + H₂O + OH + H₂O X Ś 10: 000 18 Ararrow_forwardOH Ph Br 2 1. Mg 2. CH3COCH3 3. H3O+ Br 1. 2 Mg. 2. CH3CO2CH3 3. H3O+ Br 1. Mg 2. CH2O 3. H3O+ Br 1. Mg 2. ethylene oxide 3. H3O+ at least one jarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY