
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
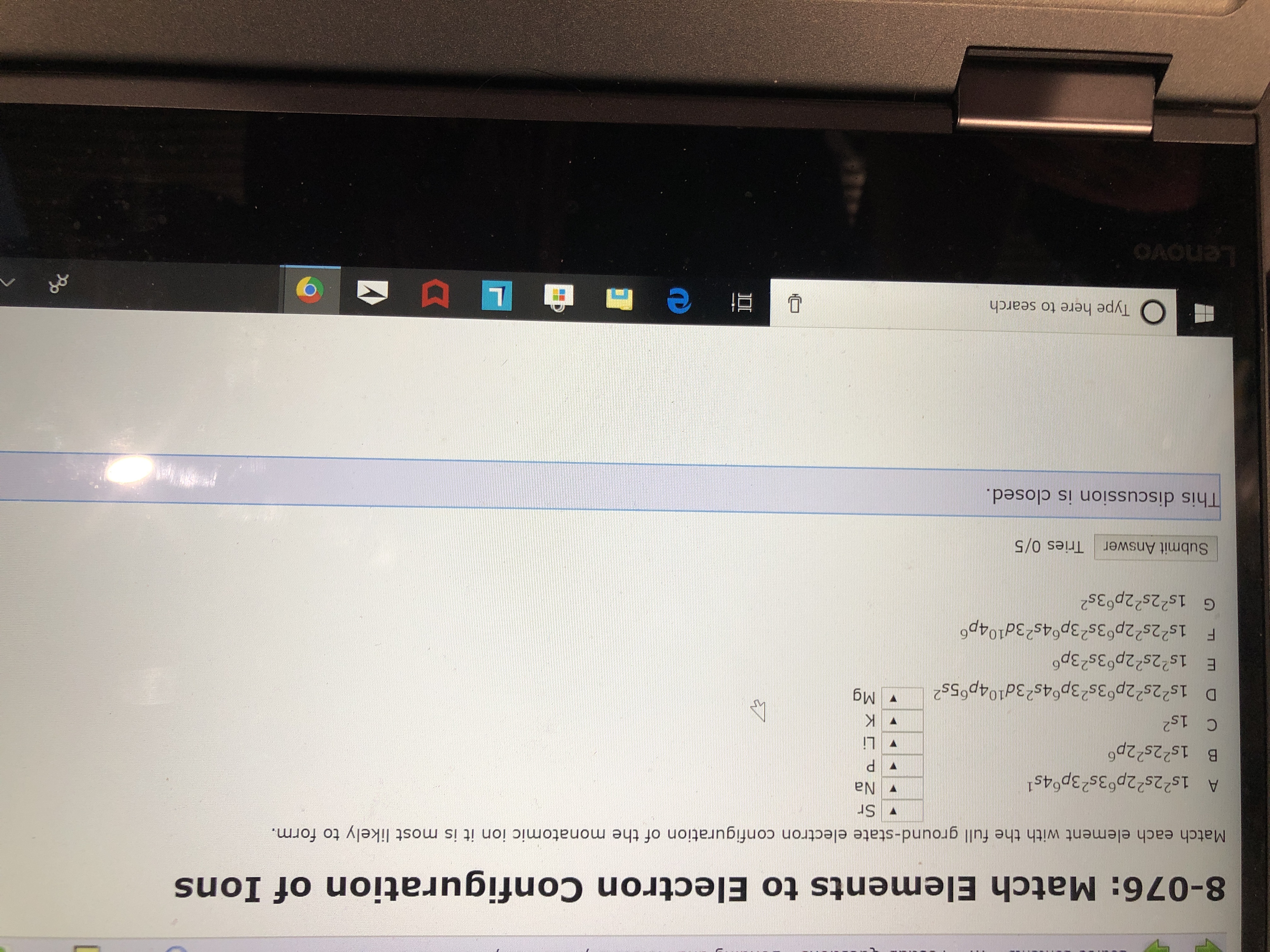
Transcribed Image Text:8-076: Match Elements to Electron Configuration of Ions
Match each element with the full ground-state electron configuration of the monatomic ion it is most likely to form.
Sr
Na
A 1s22s22p63s23p64s
B 1s22s22p6
P
Li
C 1s2
K
Mg
D 1s22s22p63s23p64s23d104p65s2
E 1s22s22p 3s23p
F 1s22s22p63s23p64s23d104p
G 1s22s22p63s2
Tries 0/5
Submit Answer
This discussion is closed.
L I
Type here to search
OAOUne
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- A1.arrow_forwardArrange the ions from largest to smallest. Br Mo6+ Rb+ Se²- Largest ionic radius Smallest ionic radius Answer Bank Y³ +arrow_forwardAtomic radius 1. increases 2. decreases Because 1. the number of electrons increases 2. the mass increases 3. the relative attraction between the protons and the outer electrons increases ο ο ο ο ο ο O 2,1 O 1,2 2,3 2,2 1,1 from left to right across a row on the Periodic Table. O 1,3 #f #arrow_forward
- Consider the theoretical electron configuration below. X. 1s² 2s²2p4 Y. 1s² 2s²2p6 3s² 3d8 Which one(s) is (are) paramagnetic? X only Y only X and Z only X and Y only Y and Z only Z. 1s² 2s²2p6 3s² 3d10arrow_forwardLithium Oxygen Fluorine Bromine Silicon Zinc electrons Electron Configuration 15/201 Orbital Diagram 2p 14 1 36arrow_forwardArrange these species into isoelectronic groups. It does not matter which group goes in which box, so long as the correct species are grouped. Lit N³- Isoelectronic group A 0²- Sc³+ Isoelectronic group B Al³+ Answer Bank P3- He Isoelectronic Ar group с Be²+arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY