
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
8.0 g of helium gas follows the process 1 to 2 and to 3 shown in Figure below. Find the volume V1, V3, P2, and T3
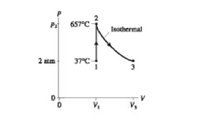
Transcribed Image Text:657°C
Isothermal
2 aim
37°C-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Please help mearrow_forward5.0 g of nitrogen gas at 20°C and an initial pressure of 2.8 atm undergo a constant-pressure expansion until the volume has tripled. Part A What is the gas volume after the expansion? Express your answer with the appropriate units. ► View Available Hint(s) V= Submit HA Value Previous Answers Units ? X Incorrect; Try Again; 5 attempts remainingarrow_forwardA pressure versus volume (pV) diagram for a system is shown in the figure. The arrows of the curve indicate the direction of the process, and the points of interest are labeled. The values for the points in the diagram are shown in the table. Volume (m³) Pressure (Pa) 2 Vo = 27.8 po = 1.37 × 10ª Vi = 20.8 Pi = 1.37 × 104 V2 = 17.4 P2 = 6.18 × 10³ V3 = 13.9 P3 = 6.18 × 10³ V4 = 13.9 P4 = 2.64 x 103 Vs = 8.87 p5 = 1.00 x 10³ Volume (m) Calculate the amount of work done on the system from 0–2 (Wm) and then for the entire curve from 0-5 (Wos). Pressure (Pa)arrow_forward
- QUESTION 16 The temperature at state A is 20.0°C, that is 293 K. During the last test, you have found the temperature at state D is 73.0 K and n = 164 moles for this monatomic ideal gas. What is the change in thermal energy for process A to D, in MJ (MegaJoules)? Your answer needs to have 2 significant figures, including the negative sign in your answer if needed. Do not include the positive sign if the answer is positive. No unit is needed in your answer, it is already given in the question statement. p (atm) 5 4 3 2 1 0 A D 1 2 3 4 B 5 → V (m³)arrow_forwardThe temperature at state A is 20.0°C, that is 293 K. During the last test, you have found the temperature at state D is 73.0 K and n = 164 moles for this monatomic ideal gas. What is the change in thermal energy for process A to D, in MJ (MegaJoules)? Your answer needs to have 2 significant figures, including the negative sign in your answer if needed. Do not include the positive sign if the answer is positive. No unit is needed in your answer, it is already given in the question statement. P (atm) 4 3 ID B +V (m) 4 5arrow_forwardThe image shows a cylinder with a movable wall (partition) and a piston. Movable partition Piston -Work Vacuum Gas What properties of matter allow the gas to expand and contract as the wall is removed and the piston moved as depicted in the image? O A The particles of a gas are close together, they completely fill the volume of the container, they are strongly attracted to each other. ©2021 Illuminate Education TM, Inc. hp -> esc #3 %24 96arrow_forward
- A large cylindrical tank contains 0.750 m³ of nitrogen gas at 22.0°C and 6.50x103 Pa (absolute pressure). The tank has a tight-fitting piston that allows the volume to be changed. Part A What will be the pressure if the volume is decreased to 0.530 m³ and the temperature is increased to 165°C? Express your answer in pascals. —| ΑΣΦ P = Submit Request Answer < Return to Assignment Provide Feedback ? Paarrow_forward4.5 mol of monatomic gas A interacts with 2.9 mol of monatomic gas B. Gas A initially has 9000 J of thermal energy, but in the process of coming to thermal equilibrium it transfers 800 J of heat energy to gas B. How much thermal energy did gas B have initially? Express your answer with the appropriate units. ► View Available Hint(s) EBi = Submit Value 4 Previous Answers Units ?arrow_forward70 J of work are done on the gas in the process shown in (Figure 1). Figure p (kPa) 300 200 100- Screenshot f i 0 V₁ 2V₁ 3V₁ 1 of 1 V Part A What is V₁ in cm³? Express your answer in cubic centimeters. ► View Available Hint(s) V₁ = Submit VT ΑΣΦ Provide Feedback B] ? cm³arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON