
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Balance the reactions first. Show all work. Report answers in proper significant figures.
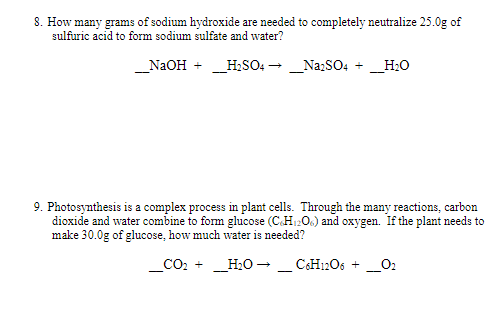
Transcribed Image Text:8. How many grams of sodium hydroxide are needed to completely neutralize 25.0g of
sulfuric acid to form sodium sulfate and water?
_NaOH + _HSO: - _Na:SO4 + _H:O
9. Photosynthesis is a complex process in plant cells. Through the many reactions, carbon
dioxide and water combine to form glucose (C.H1206) and oxygen. If the plant needs to
make 30.0g of glucose, how much water is needed?
CO2 +
_H2O - _ CH12O6 +
_O2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 7 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hydrogen chloride gas dissolves in water to form hydrochloric acid (an ionic solution). HCl(g)H2OH+(aq)+Cl(aq) Find H for the above reaction. The data are given in Table 6.2.arrow_forward9.96 Most first aid "cold packs" are based on the endothermic dissolution of ammonium nitrate in water: NH4NO3(s)NH4+(aq)+NO3(aq) H= 25.69 kJ A particular cold pack contains 50.0 g of NH4NO3 and 125.0 g of water. When the pack is squeezed, the NH4NO3dissolves in the water. If the pack and its contents are initially at 24.0°C, what is the lowest temperature that this bag could reach? (Assume that the ammonium nitrate solution has a specific heat of 4.25J g-l K-l, and that the heat capacity of the bag itself is small enough to be neglected.)arrow_forwardWhen 7.11 g NH4NO3 is added to 100 mL water, the temperature of the calorimeter contents decreases from 22.1 C to 17.1 C. Assuming that the mixture has the same specific heat as water and a mass of 107 g, calculate the heat q. Is the dissolution of ammonium nitrate exothermic or endothermic?arrow_forward
- ou place hot metal into a beaker of cold water. ol type='a'> Eventually what is true about the temperature of the metal compared to that of the water? Explain why this is true. i>Label this process as endothermic or exothermic if we consider the system to be the metal. Explain. the water. Explain.arrow_forwardWhen one mol of KOH is neutralized by sulfuric acid, q=56 kJ. (This is called the heat of neutralization.) At 23.7C, 25.0 mL of 0.475 M H2SO4 is neutralized by 0.613 M KOH in a coffee-cup calorimeter. Assume that the specific heat of all solutions is 4.18J/gC, that the density of all solutions is 1.00 g/mL, and that volumes are additive. (a) How many mL of KOH is required to neutralize H2SO4? (b) What is the final temperature of the solution?arrow_forward. A(n) _______ speeds up a reaction without being consumed.arrow_forward
- A 21.3-mL sample of 0.977 M NaOH is mixed with 29.5 mL of 0.918 M HCl in a coffee-cup calorimeter (see Section 6.6 of your text for a description of a coffee-cup calorimeter). The enthalpy of the reaction, written with the lowest whole-number coefficients, is 55.8 kJ. Both solutions are at 19.6C prior to mixing and reacting. What is the final temperature of the reaction mixture? When solving this problem, assume that no heat is lost from the calorimeter to the surroundings, the density of all solutions is 1.00 g/mL, the specific heat of all solutions is the same as that of water, and volumes are additive.arrow_forwardA 29.1-mL sample of 1.05 M KOH is mixed with 20.9 mL of 1.07 M HBr in a coffee-cup calorimeter (see Section 6.6 of your text for a description of a coffee-cup calorimeter). The enthalpy of the reaction, written with the lowest whole-number coefficients, is 55.8 kJ. Both solutions are at 21.8C prior to mixing and reacting. What is the final temperature of the reaction mixture? When solving this problem, assume that no heat is lost from the calorimeter to the surroundings, the density of all solutions is 1.00 g/mL, and volumes are additive.arrow_forwardHypothetical elements A2 and B2 react according to the following equation, forming the compound AB. A2(aq)+B2(aq)2AB(aq);H=+271kJ/mol If solutions A2(aq) and B2(aq), starting at the same temperature, are mixed in a coffee-cup calorimeter, the reaction that occurs is a exothermic, and the temperature of the resulting solution rises. b endothermic, and the temperature of the resulting solution rises. c endothermic, and the temperature of the resulting solution falls. d exothermic, and the temperature of the resulting solution falls. e exothermic or endothermic, depending on the original and final temperatures.arrow_forward
- The carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by reaction with lithium hydroxide 2LiOH(s)+CO2(g)Li2CO3(s)+H2O(l) Estimate the grams of lithium hydroxide required per astronaut per day. Assume that each astronaut requires 2.50 103 kcal of energy per day. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 2.50 103 kcal of heat, calculate the amount of CO2 produced and hence the amount of LiOH required. The H for glucose(s) is 1273 kJ/mol.arrow_forward9.58 For the reaction C2H2(g)+2H2(g)C2H6,H=136 kJ. What are the ratios that can be defined between moles of substances and energy?arrow_forwardUse the same fat described in Question 27. (a) Write a thermochemical equation for the metabolism of fat. (b) A dieter reduces his food intake by 425 nutritional calories a day (1 nutritional calorie=1000calories). How many kJ can he lose in one day? (c) How many pounds of fat are lost in one week with this diet?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning