
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:8. Construct a theoretical curve for the titration of HAC by sodium hydroxide (NaOH).
The volume of acetic acid: in the beaker is 25 ml and its concentration is 0.02 M. The
titrant is NaOH and its concentration is also 0.02 M. Calculate pH versus volume of
NaOH, which will be added in increments of 1 mL. Construct this titration curve for
35 mL. Show only the calculations at VNaoH=0, 0 < VNaOH < Ve, VNaOH=Ve and VNaOH
> Ve).
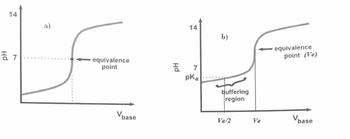
Transcribed Image Text:14
a)
금 7
equivalence
point
Vbase
Hd
14
b)
equivalence
point (Ve)
7
pka
buffering
region
Ve2
Ve
Vbase
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 6 images

Knowledge Booster
Similar questions
- Find the pH during a titration of 1.00 ml of 0.600 M analyte when a) 60.00 ml b) 120.00 ml of 0.0100 M titrant has been added then sketch the titration curve. Titrant: HI Analyte: KN3arrow_forwarda) In a NaOH and HCl titration lab: Suppose you add 40 ml of water instead of the 45 mL. How will this affect the calculations for the molarity of the HCI? (In this case, the molarity of HCl turned out to be 0.559 mol/L). While being specific. Explain why it will not matter or it will make the molarity high or it will make the molarity low. b) if you disregard the instructions to titrate until a persistent blue endpoint is reached and instead conclude the titration at the initial appearance of blue, describe the impact on the reported molarity of the CoC12 solution. Provide specific details and specify whether the reported molarity will be higher, lower, or remain unaffected.arrow_forwardNow your turn! Sketch the neutralisation curve when 40 cm³ of 0.50 moldm³ sodium hydroxide solution is added to 20.0 cm³ of 0.40 moldm³ propanoic acid (pKa = 4.9). ● Extension: Consider whether methyl orange is an appropriate indicator to find the end point of this titration MacBook Airarrow_forward
- A chemist titrates 100.0 mL of a 0.0621 M hydrobromic acid (HBr) solution with 0.6950M KOH solution at 25 °C. Calculate the pH at equivalence. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = 0 X 5arrow_forwardA scientist is attempting to recreate this titration using the same concentration of hydrochloric acid, but decides to use a lower concentration of sodium hydroxide(titrant). The original concentration of sodium hydroxide is 0.1M. How will this affect the amount of titrant needed? Explain.Correctly predicts the relative amount of titrant needed and explains.arrow_forwardA chemist titrates 150.0 mL of a 0.8461M hydrobromic acid (HBr) solution with 0.2857M NAOH solution at 25 °C. Calculate the pH at equivalence. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of NaOH solution added. PH = |arrow_forward
- 10 11 12 13 14 15 16 17 18 19 20 A chemist titrates 170.0 mL of a 0.3333 M nitric acid (HNO, solution with 0.1703M NaOH solution at 25 °C. Calculate the pH at equivalence. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the soluțion equals the initial volume plus the volume of NaOH solution added. pH = %3D Submi Continue MacBook Airarrow_forwardDetermine the Ka value Solution 1 3.98 X 10-3 M Solution 2 6.03 X 10-4 M Solution 3 1.07 X 10-5 M Solution 4 1.05 X 10-5 M Solution 5 4.68 X 10-10 Marrow_forwardA chemist titrates 150.0 mL of a 0.6793 M potassium hydroxide (KOH) solution with 0.0962 M HNO, solution at 25 °C. Calculate the pH at equivalence. Round your answer to 2 decimal places Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HNO, solution added. pH = 0arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY