
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:8 https://openvellum.ecollege.com/course.html?courseld=16914186&OpenVellumHMAC=c662ec09ac8023bd1b971c039afd0082#10001
<Chapter 8 Problem Set
Exercise 8.93
< 5 of 20 >
I Review | Constants| Periodic Table
The quantum yield of light-induced chemical reactions (called photochemical reactions) measures the efficiency of the process. The quantum yield, , is defined
as d = number of reaction events
TUmber of photona abeorbed Suppose a quantum yield for the reaction CH3XCH3 +X is o = 0.97. A cuvette containing a solution of CH,X is irradiated with 280-nm with a power
of 885 mW for 10.0 minutes.
• Part A
Assuming total absorption of the light by the sample, what is the maximum amount (in moles) of CH3X that breaks apart?
Express your answer to two significant figures.
?
mol CH,X actual
Submit
Request Answer
Provide Feedback
Next >
81%
9:40 PM
10/19/2021
Home
End
Insert
Delete
F10
&
Backspace
NumLock
6
7
8.
Y
7
8
Home
H JK L
5
Enter
B
N M
Shift
End
PriSc
Cir
Pup
PDn
44
Expert Solution
arrow_forward
Step 1
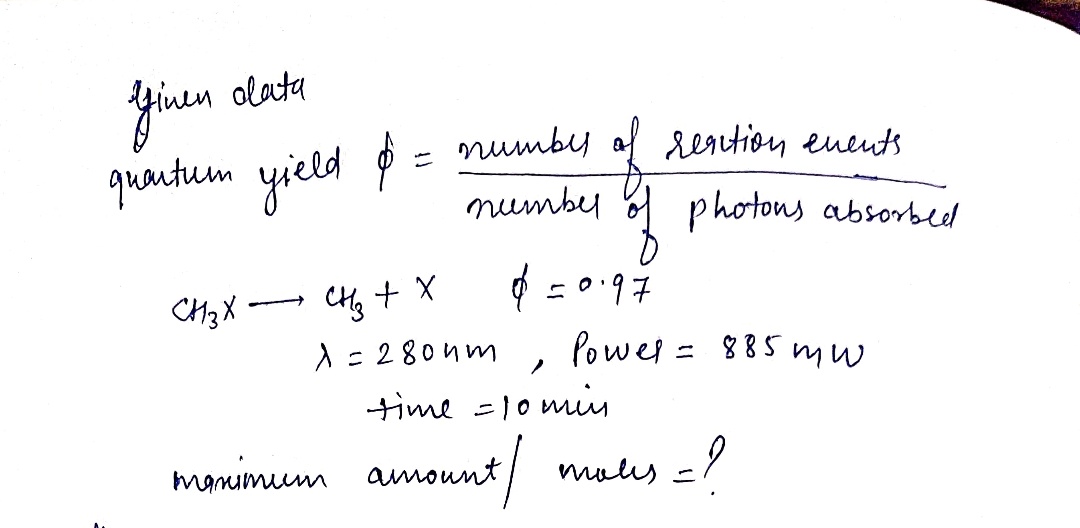
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Chrome File Edit View History Bookmarks People Tab Window Help A ALEKS - Jacqueline Hoppenrey x Mcc Chapter 4: Reactions in Aqueo x G halogens - Google Search A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQİHqRdYV_6Ux63SypJXz0Coxvwqgg4JkWI7UFmRjQiHo9r7UFmxvG2Y0ofvn27IAf2ti O SIMPLE REACTIONS Predicting the products of a combustion reaction Predict the products of the following reaction. If no reaction will occur, use the NO REACTION button. Be sure your chemical equation is balanced! CH,CH,CH,(g) + 0,(8) -0 NO ローロ REACTION Explanation Check ©2021 McGraw-Hill Education IIarrow_forwardConstants: • c = 2.9979 x 108 m/s • h = 6.626 x 10-34 J s per one photon • R = 8.314 J/(K mol) = 0.08206 Latm/(K mol) = 6.022 x 1023 particles/mol NA = RH = 1.097 x 107 m1 = 2.178 x 10-18 J . You conduct a Photoelectric Experiment and shine light with a wavelength of 5.34 x 10-7 meters onto a metal surface. Electrons are ejected with a Kinetic Energy of 79.0 kJ/mol. What is the Binding Energy (in kJ/mol) for the metal?arrow_forwardFeedback COAST Tutorial Problem What is the energy associated with the formation of 4.35 g of 4He by the fusion of ³H and ¹H? x 2.24 x 10 12 J Substance 4He 3H 1Η Mass (u) 4.00260 3.01605 1.00783 .:: See Periodic Table See Hintarrow_forward
- answer 10minarrow_forwardB Syllab y health Bb Stever y! www.h y! bulldo Post A Quest O Molec Post A Post A Ohttps: A ezto.mheducation.com/ext/map/index.html?_con=Dcon&external_browser3D0&launchUrl=https%253A%252F%252Fbsuonl Maps Bb Laboratory 6: Cell.. • YouTube Saved Chapter7-Quantum Theory and Atomic Structure i 12 Select all that apply. Only certain electron transitions are allowed from one energy level to another. In one-electron species, the change in the quantum number I of an allowed transition must be ±1. For example, a 3p electron can drop directly to a 2s orbital but not to a 2p. Thus, in the UV series, where ninal = 1, allowed electron transitions can start in a p orbital (I = 1) ofn= 2 or higher, not in an s (I = 0) or d (l = 2) orbital of n = 2 or higher. From what orbital do each of the allowed electron transitions start for the first four emission lines in the visible series (nfinal = 2)? %3D Check all of the allowed transitions: 3s 2s 3s → 2p 3p 2s 3p → 2p 3d 2s 3d 2p 4s 2s 4s 2p Graw II!H <…arrow_forwardGeneral CHEMISTRY one.. Lab 8 ( Food Dye Forensic) Please help me answer these questionsarrow_forward
- /Itemview?offset3Dnext&assignmentProblemlD=D182385223arrow_forwardnow.com/ilrn/takeAssignment/takeCovalent Activity.do?locator=assignment-take [References] Exposure to high doses of microwaves can cause tissue damage. Estimate how many photons, with A = 16 cm, must be absorbed to raise the temperature of your eye by 1.5 °C. Assume the mass of an eye is 11 g and its specific heat capacity is 4.0 J/g. K. photons Submit Answer Try Another Version 10 item attempts remaining New iCloud Terms and Conditions To use iCloud on this Mac you must accept the new Terms and Conditions. Cengage Learning Cengage Technical Support Previous Next Save and Exitarrow_forwardPick two wavelengths of light (use 3 sig figs and units of nm). Pick one from the X-ray region and one from the microwave region. Calculate the energy and frequency for each of the chosen wavelengths and discuss the relationship between wavelength, frequency, and energy.arrow_forwardQuestions lesbucional sinine52 1. List the elements tested in the flame test in order of increasing energy of the light emitted (based on the general color of the flame).arrow_forwardREPORT ATOMIC EMISSION SPECTROSCOPY 85 Jamin GoaLeler NAME Analysis of the Hydrogen Spectrum. SECTION Actual wavelength of the bright green line from the fluorescent lights, (nm) Observed wavelength of the bright green ihe from the fluorescent lights, (nm) 546 Calibration factor, (nm) Hydrogen Observed Wavelength (nm) Corrected wavelength (nm) GE4 Color Corrected wavelength (m) Initial n value Red 6.54x 10-7m372 4.86 x 10-7 m4>2 Green 488 blue 430 4.38 x 10 m 5->2 Purple 4.13 X10-7 m6->2 413 Data to be plotted for graphical method Inverse of initial na value (in decimal form) Inverse of wavelength (m-) R, (in m-1) from the slope of line plotted in the graphical method Percent error, (%) CALCULATIONS (use separate sheets to show your work) b54 x 10 4 1 esy *10 9m 52 22 32 b59 x1D-9m 21 1.arrow_forwardow Help 79% Tue 9:36 AM A docs.google.com he Roswell Incident G Physics STAAR Review - Google Sli. G Earth and Space STAAR Review -.. G Chemistry STAAR Review - Goog eview O Present S Share rrange Tools Add-ons Help Last edit was seconds ago Background Layout - Theme Transition I 1 2 I 3 I 4 1 5 6 9 I Fill in the missing information to complete the chart. Check One Box Check One Box Fill out each of these boxes. Total # of Total # Elements of Atoms Chemical Chemical Element Compound Total # of Substance Symbol Formula Molecules H2O 3 1 Cu4 CaCO3 1 Zr Ca3(PO4)2 2CO 2 4 2 speaker notes )山T EPIC MacBook Air E5 F6 F7 F8 F9 F10 F11 F12 % & * +arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY