
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What reactants are needed to proceed this reaction?
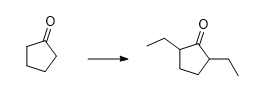
Transcribed Image Text:ů
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Select all compounds that are able to deprotonate ethanol, EtOH, to the extent that the neutralization reaction is product-favored at equilibrium. a. Na2s b. NaNH2 С. BuLi d. NaF e. NaCN f. NaCH3CO2 g. NH3 h. CH3LIarrow_forwardWrite an ionic equation to describe the formation of ions in aqueous acetic acidarrow_forwardAccording to the proposed schemes, write the equations of reactions. Be sure to write down the formulas of substances lactic acid lactide formationarrow_forward
- V. Questions 1. Correlate the number of carbon in the R group with the solubility of the acid in water.arrow_forwardNaHCO3(s) + HC2H3O2(aq) =CO2(g)+H2O(l)+NaC2H3O2(aq) Is this an oxidation or reduction reaction? Just choose one, not both.arrow_forward5. Synthesis of aspirin a. Write the sequence of reactions to produce aspirin using salicylic acid, acetic anhydrous, and phosphoric acid.arrow_forward
- What is the [H3O*1 in a 175 ml sample of 0.0629 M KOH(aq)? 9.08 x 10-13 mol L-1 O8.17 x 10-14 mol L-1 O1.59 x 10-13 mol L-1 O 2.78 x 18-14 mol L-1 6.08 x 10-13 mol L-1arrow_forwardWhat is the balanced equation for salicylic acid + acetic anhydride --> acetylsalicylic acid (aspirin) + acetic acid? When added, why is concentrated sulfuric acid a catalyst?arrow_forwardWhen setting up an organic reaction for the first time, a concentration of reactants in tens to hundreds of micromolar range (mM) is often required. How much solvent (DCM) is required to prepare a solution that has a reactant concentration of 200mM? a) 0.126mL b) 1.26mL c) 12.60mL d) 126mL e) None of the options is correct.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY