
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
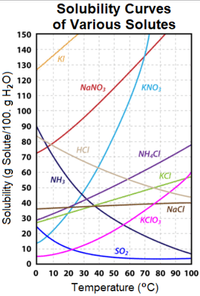
7)According to the solubility curve above, what is the maximum amount of sodium nitrate that can dissolve in 100g of water at 40 degrees C?
40 g
100 g
105 g
150 g
65 g
8) You decide to add 40 grams of ammonium chloride to 100g of water at 60 degrees C. You stir for an extremely long time. Which of the following is true?
A saturated solution is created, and around 15 more grams of solute can be dissolved.
A saturated solution is created, and around 15 grams of solute did not dissolve.
An unsaturated solution is created, and around 15 more grams of solute would be able to be dissolved, if added.
An unsaturated solution is created, and around 15 grams of solute did not dissolve.
Transcribed Image Text:Solubility Curves
of Various Solutes
150
140
Ki
130
120
NANO;
KNO
110
100
90
80
HCI
NH.C
70
60
NH3
50
40
NacI
30
KCIO;
20
10
SO2
10 20 30 40 50 60 70 80 90 100
Temperature (°C)
Solubility (g Solute/100. g H20)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 100 90 NANO, 80 70 CaCl, Pb(NO.)2 60 50 40 Naci KCI 30 20 KCIO, 10 O 10 20 30 40 50 60 70 80 90 100 Temperature (°C) How many grams are needed to create a saturated solution of Pb(NO3)2 at 30°C if you have 20 grams of Pb(NO3)2 already dissolved in a solution? Solubility (g of salt in 100 g H,O) ONYarrow_forwardA solution containing 25.0 g of protein per liter has an osmotic pressure of 8.3 mmHg at 37°C. What is the molar mass of the protein? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a b с d e 5.8 X 104 g mol-1 7.3 X 104 g mol-1 9.3X 104 g mol-1 1.2 X 105 g mol-1 3.2 X 105 g mol-1arrow_forward* What is the molarity of a solution resulting from combining 1.23 g of NaCl in 137 g water. The resulting solution has a density of 1.1 g/mL. Information that may or may not be needed: Molar mass of NaCl is 58.44 g/mol. Molar mass of H₂O is 18.02 g/mol Your Answer: Answerarrow_forward
- The solubility of heptanol, CH3(CH₂)6OH, is 0.16 g per 0.10 L. What is the molarity of the solution? 0.073 0.013 0.0073 0.133 0.16arrow_forwardhow is the solubility of a solid in a liquid affected by temperature?arrow_forwardUse the solubility curve below to answer the following questions: 150 140 130 120 110 100 NANO3 90 80 70 60 NH&Cl KCI NaCi 50 40 30 20 KCIO3 10 Ce2(SOals O 10 20 30 40 50 60 70 80 90 100 Temperature (c) Q1) As the temperature increases, the solubility of the NH3 in water Q2) The solute, NH3, is MOST likely a (increases/decreases/stays the same). (solid, liquid, or gas). Q3) How many grams of NH4CI are dissolved in 500 g of water at 90 °C? Q4) If you had 50 grams of NH4CI in 100 g of water at 40 °C, would the solution be saturated, unsaturated, or supersaturated? Q5) You heat a solution of KCIO3 to 80°C and saturate the solution. You then allow the solution to cool down to 50°C. How many grams of solute would precipitate out of the solution? NH3 per 100 g H,0 Grams of solute ONO CONarrow_forward
- Determine the freezing point (in °C) of a solution that contains 204 g of C6H1206 dissolved in 234 mL of acetic acid (density = 1.05 g/mL). Pure acetic acid has a freezing point of 16.6°C and a K = 3.90°C/m.arrow_forwardMy X OWL X CICh x Para X EA ve X nment/takeCovalent Activity.do?locator=assignment-take uiz 5.pdf Solubility of Ionic Compounds Classify each of the compounds as soluble or not soluble: Miron (III) hydroxide cobalt(II) bromide magnesium iodide Submit Answer TREMENTS OCT W Math 122 Quiz 5.docx 12 tv (41 X [Review Topics] [References] Use the References to access important values if needed Retry Entire Group 9 more group attempts remaining PR F22-Chem-103x....pdf 2 Mic x A ΕΟ S Fel-F22-Charrow_forwardUnderstanding how solubility varies with temperature and pressure.arrow_forward
- A saturated solution of a compound contains .15g of solute in 100 g of water. Is a second solution containing .05 g of solute dissolved in 25 g of water unsaturated, saturated, or supersaturated?arrow_forwardHow many grams of chlorine are in 250 milliliters of a 1.50 M MgCl2 solution? Hint: the solubility of MgCl2 is high enough for all of it to completely dissolve. You can assume that the molar mass of dissolved Cl- ions is the same as molar mass of Cl atoms you can look up in the periodic table.arrow_forwardWhich of the following phrases best describes the term solubility? the ability of a solvent to dissolve in a solute the ability of a solute to dissolve in a solvent the maximum amount of solute that will dissolve in solvent at a given temperature the maximum amount of solvent that will dissolve in a solute at a given temperature the amount of solvent that dissolves in a solute for each 10oC rise in temperaturearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY