
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't use hend raiting
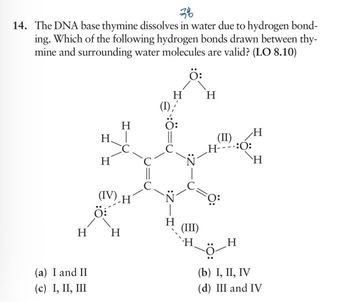
Transcribed Image Text:76
14. The DNA base thymine dissolves in water due to hydrogen bond-
ing. Which of the following hydrogen bonds drawn between thy-
mine and surrounding water molecules are valid? (LO 8.10)
H
H|
H
(IV) H
H
H
(I)'
(II)
H
H----:O:
H
H
H
H
(III)
H
H
(a) I and II
(c) I, II, III
(b) I, II, IV
(d) III and IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Dinitrogen trioxide decomposes to NO and NO2 in an endothermic process (AH = + 40,5 kJ/mol). 4. N2O3 (g) = NO (g) + NO2 (g) Predict the effect of the following changes on the position of the equilibrium; that is, state which way the equilibrium will shift (left, right, or no change) when each of the following changes is made. Motivate each prediction fully. (a) Adding more N2O3 (g) (b) Adding more NO2 (g) (c) Decreasing the temperature (d) Increasing the container volumearrow_forwardEstimate ΔrH° for forming 2 mol ammonia from molecular nitrogen and molecular hydrogen. Is this reaction exothermic or endothermic?arrow_forward7-45 (Chemical Connections 7C) A painkiller—for example, Tylenol—can be purchased in two forms, each containing the same amount of drug. One form is a solid coated pill, and the other is a capsule that contains tiny beads and has the same coat. Which medication will act faster? Explain.arrow_forward
- What will happen to the equilibrium, NaHCO3(s)Na2CO3(s) + CO2(g) + H2O(g);Ho = 136 kJ when: (a) the temperature is decreased(b) the volume of the container is increased (c) water vapor is removed(d) additional Na2CO3 is added(e) pressure of CO2 is increasedarrow_forward8:59 l LTE O AWhy are iron oxides in rocks evidence for prehistoric photosynthesis? (1 O Iron oxides form when oxygen, a product of photosynthesis, is in the atmosphere O Iron oxides form when cyanobacteria use carbon dioxide for photosynthesis. O Iron oxides form when carbon dioxide, a reactant of photosynthesis, is in the atmos O Iron oxides form when cyanobacteria produce oxygen via photosynthesis. O Type here to search escarrow_forwardFor a reaction with AH < 0, which of the following must be true? O The bonds broken are stronger than the bonds formed. O The bonds broken are weaker than the bonds formed. O All bonds are broken heterolytically. O All bonds are broken homolytically.arrow_forward
- (Q99) Consider the formation of sulfur dichloride gas from gaseous sulfur and chlorine gas: S8 (g) + 8 Cl2 (g) 8 SCI2 (g). If the equilibrium concentrations at a certain temperature are as follows, what is the value of the equilibrium concentration (KJ? (3 sf) S8 (g) = 0.356 M Cl2 (g) = 0.799 M SCI2 (g) = 0.851 Marrow_forwardAcetaldehyde (CH3CHO) is an important chemical both industrially and biologically. For instance, it is a (somewhat toxic) intermediate in the body's metabolism of ethanol into acetic acid, and thus is possibly implicated in the "hungover" symptoms of someone who has had too much to drink the night before. In aqueous solution, it establishes an equilibrium with a hydrated form, shown below. CH3CHO (aq) + H2O (l) <--> CH3CH(OH)2 (aq) You start with an aqueous sample, already at equilibrium, with the CH3CH(OH)2 (the hydrated form) at a concentration of 2.60 M. You have no information about how much, if any, of the anhydrous form (CH3CHO) is initially in the flask. If you add 2.0 M of CH3CHO to the reaction flask, and as the equilibrium is being restored the amount of CH3CH(OH)2 changes by 1.13 M, what is the final amount of CH3CHO?arrow_forwardगड्डेडेजग mo కం కా cooction the manymoles of copor (1) ulAdearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning