
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
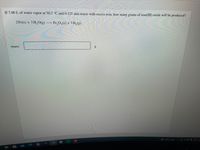
Transcribed Image Text:If 7.00 L of water vapor at 50.2 °C and 0.121 atm reacts with excess iron, how many grams of iron(III) oxide will be produced?
2 Fe(s) + 3 H,O(g) → Fe,O,(s) + 3 H,(g)
mass:
g
54F Haze
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many moles of sodium will react with water to produce 4.5 moles of hydrogen in the following reaction? 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)arrow_forwardHow many moles of oxygen are needed to burn 6.022 x1024 molecules of NH3?arrow_forwardWhat mass of Cu(IO3)2 can be formed from 0.650 g of CuSO4 · 5H2O? What mass of KIO3 is needed to convert the copper in 0.2750 g of CUSO4 - 5H2O to Cu(IO3)2?arrow_forward
- How much oxygen (in grams) will form from 30.2 g of N2O5? 2N2O5(s) → 4NO2(g) + O2(g)arrow_forwardWhat coefficient is placed in front of O 2 to complete the balancing of the following equation? C 5H 8 + O 2 → CO 2 + H 2O57391arrow_forwardAutomakers are always investigating alternative reactions for the generation of gas to inflate air bags, in part because the sodium produced in the decomposition of NaN3 presents safety concerns. One system that has been considered is the oxidation of graphite by strontium nitrate, Sr(NO3)2, as shown in the equation below. 5 C(s) + 2 Sr(NO3)2 (8)→ 2 SrO(s) + 2 N₂(g) + 5 CO₂(g) Suppose that a system is being designed using this reaction, and the goal is to generate enough gas to inflate a bag to 63 L and 1.4 atm at 23°C. What is the minimum mass of graphite that should be used in the design of this system? g C(s)arrow_forward
- When atomic phosphorous (P) and oxygen are directly combined and react, P4O10 is produced. Write the balanced chemical equation for this reaction. What is the coefficient of oxygen?arrow_forwardCalcium hydride reacts with water to produce hydrogen gas according to the following reaction: CaH2(s) + 2 H2O(l) ----> 2H2(g) + Ca(OH)2(aq) This reaction is used to generate hydrogen gas to inflate air bags in cars and life rafts on boats and for similar uses where a simple compact means of hydrogen generation is necessary. Assuming complete reaction with water, how many grams of calcium hydride are required to fill a raft to a total pressure of 2.0 atm at 25oC if the volume of the raft is 1,000 L?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY