
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
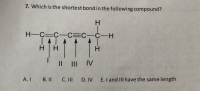
Transcribed Image Text:7. Which is the shortest bondin the following compound?
H.
H-C=C-C=C_C-H
111
H
H
H
Il III IV
A. I
B. II
C. III
D. IV E.I and III have the same length
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 8. CH3CCH (propyne or methylacetylene) Projection Drawing: Perspective Drawing: lives a. Predict the angel HCC (the first carbon) CCC. b. Predict the molecular geometry around each carbon atomarrow_forwardd. You are given a mixture of three compounds (A, B, C, below). OH CH3 OCH3 В A C a. Rank the three compounds from least to most polar. least polar most polararrow_forwardPlease don't provide handwriting solutionarrow_forward
- 3. Based on 00 a. Which compound is the least likely compound to be stable PH3 PC13 PC14 PC15 b. Which compound is most likely to be stable? B2 B₂H6 BCN BO3 c. A number of concatenated sulfur forms are known, such explanaarrow_forwardComplete the fourth resonance structure to determine which bond is shortest? Which compound will react fourth fastest with B-eBr ? d. e. F 14. Complete the fourth resonance structure to determine which bond is shortest? 15. Which compound will react fourth fastest with Br₂/FeBr3? a. b. d. -OH b darrow_forwardDetermine the geometry around all second-row elements in each compound drawn as a Lewis structure with no implied geometryarrow_forward
- Which orbitals overlap to create the C-C a-bond in ethyne? Select one: O a. sp and sp O b. sp ands O c. sp? and sp? d. s and s e. sp and sparrow_forward1. For each of the following reactions, draw the bond line structure(s) of the product(s): OH 1) MeMgBr 2) H3O+ 1) TsCl, py OH 2) NaOEt conc. H₂SO4 heatarrow_forwardPls ans botharrow_forward
- What would be the approximate H-C-H bond angle in CH3OH? (In other words, what is the approximate angle formed between two of the C-H bonds in CH3OH?) O90 degrees O 109.5 degrees O 120 degrees 180 degreesarrow_forwardIdentify which ONE or MORE of the following molecules provided below are polar (note that the structures may not be represented in the correct molecule geometry). F S- H H Structure 2 Structure 3 O O F-Si F Structure 1 F ai CI CI Structure 4 a. Structure 1, Structure 2, Structure 4 b. Structure 3, Structure 4 C. Structure 2, Structure 3, Structure 4 d. Only Structure 2, Structure 4 e. Only Structure 4 S=C=S Structure 5 (.arrow_forward14. The carbon with the largest delta value (the most deshielded) is يعلمهم ЊС a. a b b. b C. Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY