
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
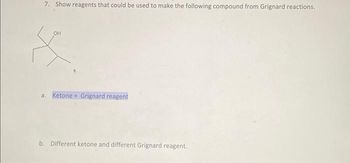
Transcribed Image Text:7. Show reagents that could be used to make the following compound from Grignard reactions.
OH
X
a. Ketone + Grignard reagent
b. Different ketone and different Grignard reagent.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Match the correct reagents and products where necessary on the reaction map:arrow_forward64. What reagent(s) will accomplish this conversion? a. EtMgBr followed by an acid wash b. EtLi followed by an acid wash Et2 CuLi followed by an acid wash с. d. Et3SiCl followed by an acid washarrow_forwardI need help with the ones that are circled. Looking for the reagents necessary for the following transformations. Thank youarrow_forward
- 1. Propose multi-step syntheses to make the products shown below. Separate the steps with numbers. You should think about the product after each step you propose to make sure the scheme will work. Use the summary of reactions at the end of Ch 8,9, 10, 11, 17 to help you. Count the number of carbons in the reagents and products. A) OH .CI B) ??? HO. C) H2 .C ??? CH3 CH3CCCH3 H3C `CH3 Ноarrow_forwardIdentify the correct reagents and conditions to perform this chemoselective reaction: H ရှုံး H Select one: Me e. Reagent(s) and conditions? a. NaBH4 b. BH3 THF Pd/BaSO4, H₂ (1 atm.), quinoline, Pb(OAC)2, MeOH d. Pd/C, H2 (1 atm.), MeOH DIBAL Me H H OHarrow_forward10. Consider the below reaction between the acetylide ion and methanol. Draw the conjugate acid that is formed as a product in this reaction.arrow_forward
- Draw the products of the reaction shown below. Ignore inorganic byproducts. O₂N. cat. H₂SO4 O HNO3 (1 equiv) Drawingarrow_forwardWhich reagent(s) is/are needed to perform the following reaction (choose all that apply).CHOOSE ALL THAT APPLY a. H2O b. H2 c. Pt d. NaOH e. K2Cr2O7 f. H+ g. Cu^2+arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY