
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
What is the major product of the following reaction? Please explain why the correct answer is letter C. Include detailed steps and a drawing explaining the mechanism of the reaction.
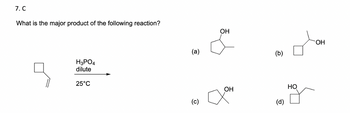
Transcribed Image Text:7. C
What is the major product of the following reaction?
H3PO4
dilute
25°C
(a)
(c)
OH
(b)
OH
OH
(d)
HO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- 2) Draw the missing reactants or products. (A) CH,Co0° N CH,COOH CH1,Br 1 (B) OH Major product (C) CH,0Na CH,OH (D) Na CN 25 °C in rtahle confimirationarrow_forwardWhat is the major product of the following reaction? OH oc | (1) BH3 (2) H₂O₂, OH-arrow_forward6. What is the major organic product generated in the reaction below? (A) CI OH (B) A.IOH C Cl₂ / H₂O (C) ||||C OH (D) OH CIarrow_forward
- What is the final product (B) of the following reaction? CH3 Br2 1. KCN light → B 2. H30*, heat CH3 CO2H CO,H CH3 CH2CO2H (a) (d) `CO2Harrow_forwardWhat type of reaction occurs when a quantity of water is added to cyclohexene in the presence of an acid catalyst that results in the formation of an alcohol? a) Substitution reaction Ob) Addition reaction c) Esterification reaction d) Elimination reactionarrow_forwardDraw structural formulas for the major organic product reactionarrow_forward
- Which is the major product of the reaction below? NaCN CN CH3CN (A) (B) OH CH2 HO OH (D) (C) CN он NC Compound B Compound C Compound A Compound Darrow_forwardWhat is the major product of this reaction? 1) xs LIAIH4 2) Hо H (B) НО. (A) (C) HO. (D) HO H. HO, HO HO (A) (B)arrow_forwardGive the major product(s) of the following reaction. 1) KMNO4, OH", heat ? 2) H30* A OH Но C D 오 Barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
