
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
None
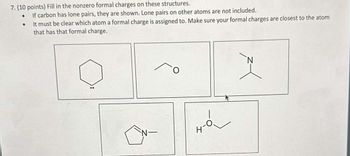
Transcribed Image Text:7. (10 points) Fill in the nonzero formal charges on these structures.
•
If carbon has lone pairs, they are shown. Lone pairs on other atoms are not included.
•
It must be clear which atom a formal charge is assigned to. Make sure your formal charges are closest to the atom
that has that formal charge.
на
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- ▼ Part B 2NO3(aq) +8H(aq) + 6e 3Fe(s) + 2NO3 (aq) +8H+ (aq) 3Fe(s)→→3Fe²+ (aq) + 6e →>> 2NO(g) + 4H₂0 (1) →3Fe²+ (aq) + 2NO(g) + 4H₂O(1) IVE ΑΣΦ Calculate El using the tabulated standard electrode potentials at 25 °C. cell Express your answer in volts to three significant figures. Eeell= 1.21 SWE Submit Previous Answers Request Answer 2+ X Incorrect; Try Again; 4 attempts remaining ? V stontiol buoubtrooting the electro naarrow_forwardthe formula for change in temoerature is final-intial right. am i correctarrow_forwardOrder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. relative pH of 0.1 M aqueous solution species H20 4 H,PO4 HNO, (Choose one) NO2 7 OH (Choose one) HF (Choose one) ▼arrow_forward
- arrow_forwardSelect the single best answer. Consider the steps in coal gasification: C(coal) + H₂O(g) → CO(g) + H2(g) CO(g) + H₂O(g) → CO2(g) + H2(g) CO(g) + 3H2(g) → CH4(g) + H2O(g) AH'rxn = 129.7 kJ AH°rxn = -41 kJ AH'rxn=-206 kJ Identify the overall reaction for the production of methane, CH4.arrow_forwardGiven the following data: 2 C6H6(l) + 15 O2(g) → 12 CO2(g) + 6 H2O(l) ΔG0= -6399 kJ C(s) + O2(g) → CO2(g) ΔG0= -394 kJ H2(g) + ½O2(g) → H2O(l) ΔG0= -237 kJ Calculate the ΔG0rxn for the reaction 6 C(s) + 3 H2(g) → C6H6(l)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY