
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:7
bints
eBook
O
References
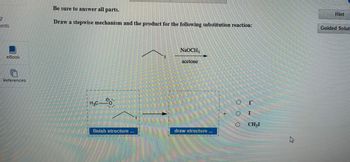
Be sure to answer all parts.
Draw a stepwise mechanism and the product for the following substitution reaction:
Hint
Guided Soluti
H.C-
NaOCH3
acetone
finish structure
draw structure
**
OOO
I
I
CHI
۵
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- :) Predict the NAME, STRUCTURE and STEREOCHEMISTRY of the BEST ORGANIC REACTANT or the MAJOR ORGANIC product for the following reactions: CH2 МСРВА, НзО* meso 3,4-hexane diol FoH H+OH CHCH a) (name, str, stereochem) ? HCI b) 3-methyl 1-pentene - ? (name, str, stereochem) c) methylcyclohexane ----Br2, hv------? (name, str, stereochem) 40H d) ? (name, str., stereochem) Os04, water----meso cyclohexane 1,2-diol ----arrow_forward34) A mixture of cis- and trans- 1,2-dimethylcyclohexan-1-ol is heated in concentrated H3PO4. Which of th following is predicted to be the major alkene product(s)? conc. HзPОд OH ??? (cis- & trans-) (-H20) a) c) d) b)arrow_forward1. Give IUPAC names fro the following alkyl halides: (a) CH3CH2CH2CH2I (b| (c) CH3 CH3 CH3CHCH2CH2CI BrCH2CH2CH,CCH28 ČH3 (d) CH3 le) ! CH,CH2CI (d) Br CI CH3CH2CH2CI CH3CHCHCH2CH3 CH3CHCH2CH2CHCH3 CIarrow_forward
- Draw the major organic product of the reaction shown below. O Ca OH **** + HBr • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. - ] Jn [1 ChemDoodleⓇ 5barrow_forwardComplete the following reactions by identifying the majority product(s) or the reaction conditions that are missing. No mechanism is neededarrow_forwardN(CH2CH3)3 + HNO3 --------> a.) rewrite the reaction using bond-line structure of reagents and products of the reaction b.) supply the curved arrows explaining the mechanism of the reactionarrow_forward
- Dehydration of 1,2,2-trimethylcyclohexanol with H2SO4 affords 1-tert-butylcyclopentene as a minor product. (a) Draw a stepwise mechanism that shows how this alkene is formed. (b) Draw other alkenes formed in this dehydration. At least one must contain a ve-membered ring.arrow_forwardCH3 CH3 CH3 CH;=ĊCH,CHCH;OH H2SO4, H3C H3C Referring to the above reaction: 1. Which atom does the H2SO4 first remove? 2. Identify the reaction if it's hydrogenation, hydrohalogenation, etc. 3. Propose a mechanism for the reaction, using the arrow formalism to indicate the flow of electrons.arrow_forward3. What is the major organic product obtained from the following sequence of reactions? [4] 1. (CH3)2CHCH2MgBr 2. H₂O OH Et₂O OH || CrO3 H2SO4 IV A) I B) II C) III D) IVarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
