
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
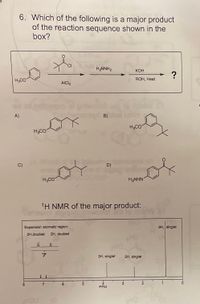
Transcribed Image Text:6. Which of the following is a major product
of the reaction sequence shown in the
box?
H2NNH2
КОН
of
H3CO
ROH, Heat
AICI3
A)
H3CO
H3CO
D)
H3CO
H2NHN
1H NMR of the major product:
Expansion aromatic region:
9H, singlet
2H,doublet
2H, doublet
CH
3H, singlet
2H, singlet
PPM
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major product for each of the following reactionsarrow_forwardQ6. Which position will be attacked most readily by the nitronium ion (NO₂+) when the compound below undergoes nitration with HNO3/H₂SO4? H I II \V III IVarrow_forwardIV. Propose a mechanism for the following transformation 12 HOarrow_forward
- 8) Predict the major product of the following reactions. a)I 2 b) п c) II d) IV e) V ОН I Н Н ОН- Н30* heat (-H2O) H ОН II ОН NaBH4 CH₂OH ? (Z and E) III (Z and E) OH по ву ОН IV Н ОНarrow_forwardWhich compound is the dominant product of the reaction shown below? (1) NaH/THF -SH (2) Br C. Br Compound C Compound A Compound B O Compound Darrow_forward13). What is the major product of the following two reactions? H₂ H3C H3C NH (A) H3C ОН H3C NH₂ OH-, then H2O (D) H3C H₂ (В) CH3 ? H3C H3C (E) ОН NH (C) CH3 -CH₂arrow_forward
- presented by Macmillan Learn Draw the product of the transformation shown by the fishhook notation. Include all hydrogen atoms and nonbonding electrons. 20 4 $ H3C Jean CH₂ R F V H3C 999 ODD FA % 5 T G F B 6 MacBook Pro Y H & 7 N ↑ 80 F7 U 8 M I K Draw the product. Select Draw Rings More ////// C ( 9 H C DD F9 V O ) O J F10 P ^. H Br - F11 { command option سال [ + F12 Erase (arrow_forwardIdentify the best reagents the following reaction. CH3 CH3 O H2O/H О он ? O H2O/Peroxide о 1. ВН3/ 2. НО, Н2О2, Н20 0 BH3 ОНarrow_forward5. What is the product of the reaction shown? CH3OCH₂ 0 OCH3 (A) CH3OCH2 H CH3O (B) HOCH2 Н H CH3O Н (C) HOCH2 ОН Н H H Н CH3O (D) HOCH2 Н Н О Н 0. ОН H Н OH Н Н Н H OCH3 ОН Н OCH3 OCH3 Н OCH3 OCH3 T Н H2O H+arrow_forward
- 12) Explain whhy compound 2 is a much weaker base than compound 1 (approximately 1000 tirnes less basic) O Steric effect and resonance will decrease the charge density on N atom in compound 2 O Resonance and induction effects will decrease the charge density on N atom in compound 2 O Inductive and resonance effects will decrease the charge density on N atom in compound 1arrow_forwardWhat reagent is needed to carry out the following transformation? HO COOH 4 HO CN ? 2. H3O* 1. LAH OH3O+ Oa) 03, b)DMS OSO4, NMOarrow_forwardwhich newman projection displays the proper antiperiplanar orientation for an E2 elimination of the reaction sequence belowarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY